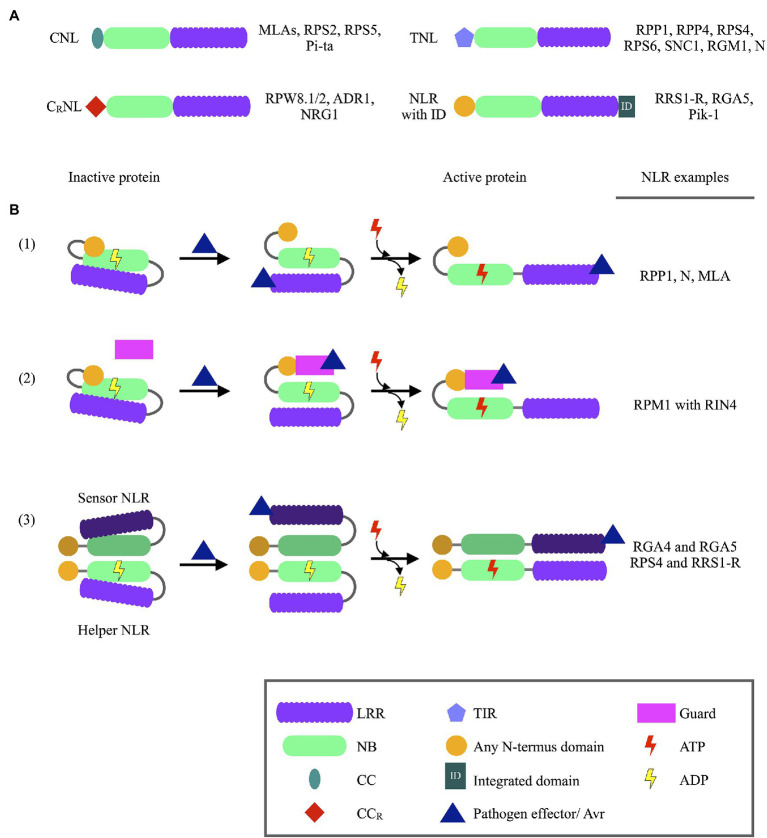
Figure 1.
Schematic representation of NLR protein domains and NLR activation, together with NLR protein examples. (A) Different structures of NLR proteins identified in plants, with NLR protein examples listed on the right of each schematic. (B) Models of Nucleotide binding-Leucine rich repeat (NLR) protein recognition of pathogen Avirulence (Avr) proteins. (1) Direct recognition of pathogen Avr protein through binding to LRR domains of NLR proteins. Avr recognition is followed by the exchange of ADP with ATP at the Nucleotide binding (NB) domain, which activates the protein and downstream immune responses. (2) Pathogen Avr proteins may bind to guard proteins which are under the surveillance of NLR proteins. Once Avr binding is recognized the NLR protein is activated through the binding of ATP, ultimately leading to immune response activation. (3) Avr recognition by NLR dimer pairs occurs when an Avr proteins binds to a sensor NLR. Structural changes of the sensor NLR induced by the Avr activates a helper NLR, subsequently leading to immune response activation. The binding of ATP to the sensor NLR is not required for NLR function (ADP—Adenosine diphosphate; ATP—Adenosine triphosphate; CC—Coiled coil; CCR—Coiled coil domain with integrated RPW8 domain; LRR—Leucine rich repeat; NB—Nucleotide binding; TIR—Toll/interleukin-1 receptor).