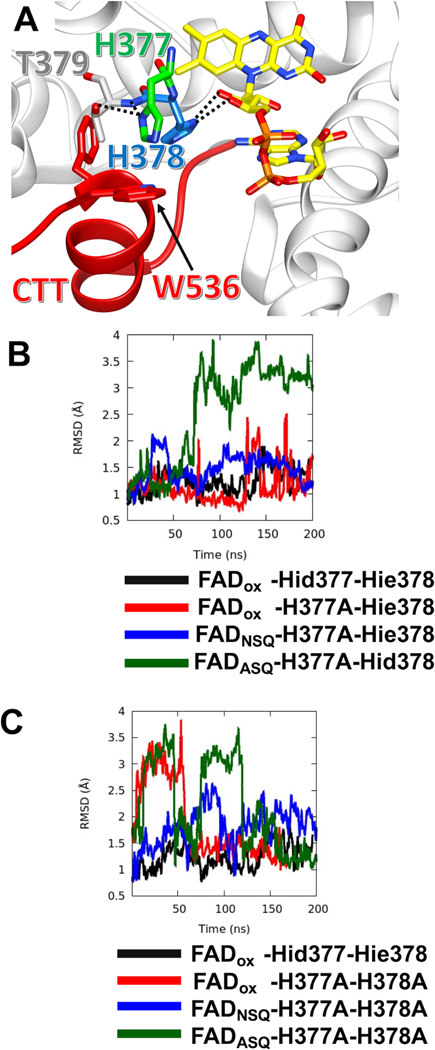
Figure 5: MD Simulations reveal varying stabilities of the CTT in CRY variants.
(A) Local bonding environment of His377 and His378. His377 (green) makes hydrogen bonding contacts with the amide nitrogen of the backbone and the hydroxyl group of Thr379; His377 also engages in edge-on van der Waal’s interactions with the sidechain of Trp536 of the FFW (red). While His378 hydrogen bonds to the ribosyl oxygens of the FAD. (B) The running average of CTT displacement with respect to the crystal conformation (closed state) is monitored over the course of various MD trajectories. Deviations in CTT conformation are observed to varying extents with the H377A variant, depending on flavin redox state. FADox – CRY with an oxidized isoalloxazine ring; NSQ – the flavin neutral semi-quinone; ASQ – the flavin anionic semiquinone; Hid – His with N1(d) protonated; Hie – His with N3(e) protonated; Hip – His with both N1(d) and N3(e) protonated. CTT conformational stability shown relative to Hid377/Hie378 for comparison (black line). The running average is calculated over a window of 400 ps. (C) MD simulations of CTT displacements in the H377A/H378A variant as a function of flavin redox state. Nomenclature as in (B).