Abstract
Extracellular vesicles (EVs) are receiving increasing attention for their role in spreading both beneficial and harmful information during cell–cell communication. The complexity and heterogeneity of the origin of EVs make integrated molecular, structural, and functional studies extremely challenging but necessary at the same time. In fact, a comprehensive interdisciplinary approach is needed to correlate the features of EVs, target cells/organs, and the pathophysiological outcomes exerted by the EVs’ actions. Based on these premises, after introducing a brief state-of-the-art outline on the current analytical approaches exploited to characterize EVs, this review aims to highlight the effectiveness of those studies where authors put in correlation the diverse EV data collected from different points of view. Although these examples are still just a few, they still represent an excellent starting point to be taken as a reference in the perspective for improving the correlation among EV-related clinical aspects. Of course, to fully reach this goal, several points need to be further improved and developed. Undoubtedly, new avenues in diagnostic, prognostic, and therapeutic applications by EVs will be initiated by integrative strategies, combining biophysical approaches, high-throughput omics technologies, and computational models.
1. Introduction
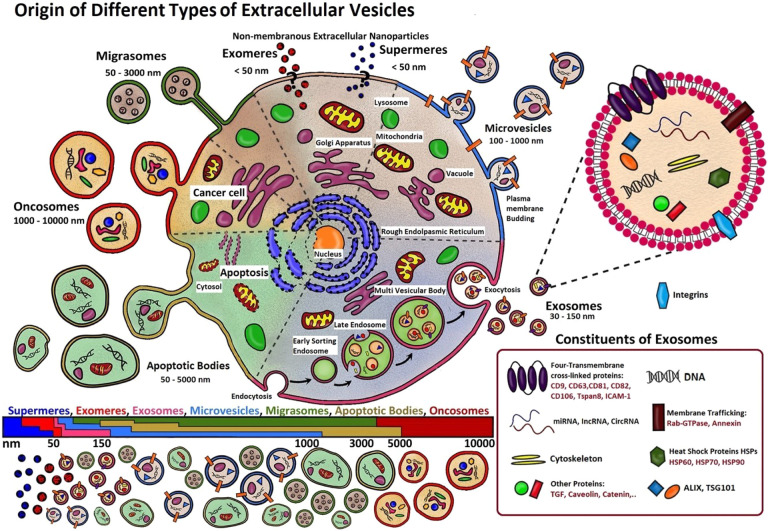
Extracellular vesicles1 (EVs) are particles of nanometer dimensions (≤1 μm) harboring a functional cargo including nucleic acids, proteins, lipids, and metabolites. They are secreted by cells through direct budding from the plasma membrane or by endosomal machinery. Based on the current knowledge, classification of EVs is typically based upon their size, cellular origin, biological function, and/or mechanism of biogenesis. In fact, different pathways lead to different EVs, with different structures and cargos. Following their size biogenesis correlation, they can be divided into two main categories: microvesicles (MVs), which are heterogeneous in diameter (∼100–1000 nm), and exosomes, which are smaller (30–150 nm), as summarized in Figure 1. However, other types of EVs also exist, including migrasomes, apoptotic bodies, oncosomes, as well as the most recently discovered supermeres2 and exomeres.3
Figure 1.
Schematic representation of the different EV subpopulations. For a long time, size was the main characteristic used to classify EVs, but contradictory results created the need for additional criteria as markers related to their biogenesis. Exosomes are the small EVs mostly originating from multivesicular bodies (MVBs) in the cell cytoplasm, formed by inward invagination of membranes of late endosomes and successive release into the extracellular space through fusion with the plasma membrane, whereas large EVs (MVs, migrasomes, oncosomes) are mainly constituted by vesicular budding from their originating cell membrane, with each type acting for a different functional purpose. Apoptotic bodies, on the contrary, originate from a cell which is in apoptosis. Moreover, the exact biogenesis of the recently discovered nonmembranous nanoparticles, exomeres and supermeres, is still debated.
The first observation of EVs dates back to 1946, when Chargaff and West observed platelet-derived particles in normal plasma; a comprehensive review of the history of EVs authored by Couch et al. was recently published.4 Briefly, a few studies were performed in the 1970s and 1980s to investigate their nature, and during those years, EVs were essentially considered to be the recycling bin of cells. In 1996, Raposo et al. could finally demonstrate the functionality of these vesicles as stimulating agents of immune response. By specifically naming the isolated vesicles as exosomes, Raposo proved that exosomes isolated from both human and murine B lymphocytes could provoke antigen-specific MHC class II-restricted T cell responses, suggesting their role in antigen presentation in vivo. Since then, the importance of EVs as intercellular communication systems has been more and more recognized up to today’s wide and interdisciplinary interest.
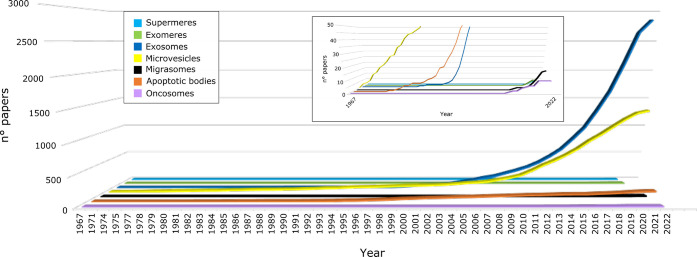
Following the great interest in EVs, the number of papers published in the last 10 years has robustly increased, and several works have demonstrated that EVs are secreted under different conditions by a variety of cells, ranging from mammals to plants, where the presence of EVs has been recently debated and demonstrated. Noteworthy, most efforts have been concentrated on exosomes, MVs, and apoptotic bodies that represent the types of EVs mostly investigated, as indicated in Figure 2.
Figure 2.
Cumulative number of papers focusing on different EV populations published from 1967 to April 2022 (collected from www.pubmed.ncbi.nlm.nih.gov). The papers were selected by the presence of EV subtype (exosomes, microvesicles, apoptic bodies, migrasomes, oncosomes, supermeres and exomeres) in their title.
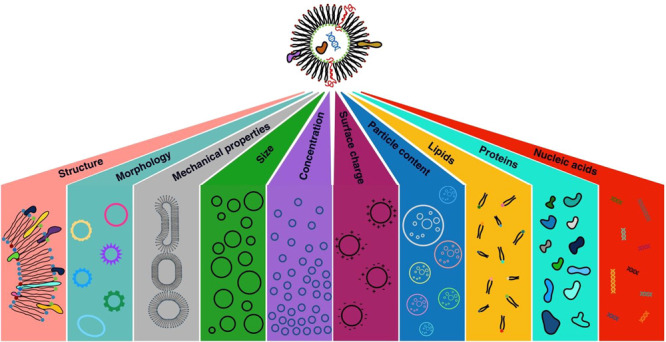
In terms of heterogeneity, EVs differ not only in their size, lipids, proteins, and nucleic acids content but also in their morphology, mechanical properties, surface charge, and density. Due to the EVs’ circulation capability for reaching proximal and distal compartments, they are vital players in the cellular communication.5 In this scenario, EVs can interact with target cells, directly altering their physiology or transferring surface receptors and cargos of bioactive molecules.6 Cell–cell communication is an essential process in living organisms, and its dysregulation may be responsible for various diseases. The EVs’ heterogeneous molecular cargo and its role in immunomodulation25,27 have inspired researchers to find the link among EVs, the immune system, and tumor development and progression.5 Yet, the link between EVs and the immune system and the role of EVs, in general, has been related to plenty of diseases, including metabolic, neurodegenerative, and cardiovascular ones, so that in parallel, due to their presence in biological fluids, their clinical relevance for diagnostic and prognostic purposes is rapidly increasing as is their relevance as therapeutic targets and agents.7 Indeed, it is more and more evident that the EVs’ chemicophysical properties correlate with the physiopathological state of their originating cells, tissues, and organisms as “messages in the bottle”: they could be used to discover multiple biomarkers related to the different tissues and diseases.
Systematic studies on EVs are fundamental for a deeper understanding of the intricate network of intercellular communication and the complex pathophysiological roles of EVs as communicasomes.1 Moreover, cell-to-environment communications are essential processes in both unicellular and multicellular organisms, including humans. In fact, recently, increasing attention has been dedicated to the EVs as interkingdom communication mediators.
To shed light on cellular communication mechanisms mediated by EVs, as well as on their chemicophysical features, most studies rely on analytical methodologies for providing multifaceted information on the EVs’ molecular cargo along with measurements for their size and morphological and mechanical properties.
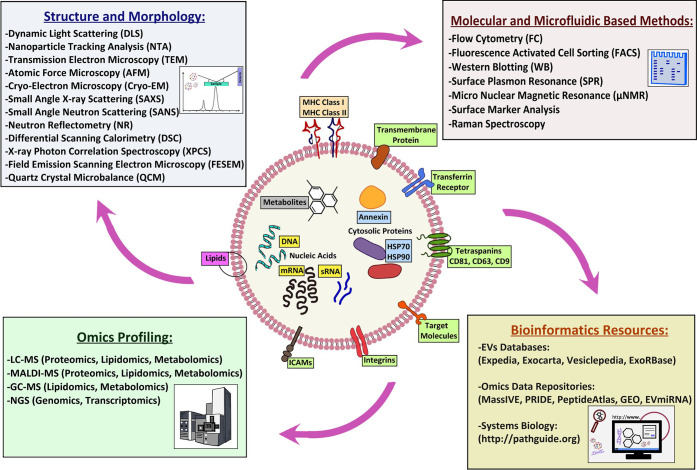
Techniques such as light scattering (LS), nanoparticle tracking analysis (NTA), atomic force microscopy (AFM), cryogenic electron microscopy (cryo-EM), scattering and reflectometry of X-rays and neutrons, and vibrational spectroscopies allow the collection of information on the size, structure, mean content, and morphology of EVs,2,3,8−13 whereas others such as Western blot analysis, polymerase chain reaction (PCR), next generation sequencing (NGS), and mass spectrometry (MS) allow a comprehensive description of their molecular content1−3,6,14−19 (see Figure 3).
Figure 3.
Diverse techniques used for investigation of EVs. Their combined application can be exploited for a more comprehensive understanding of their characteristics/function relationship.
Although the above-mentioned techniques are routinely used by the scientific community, until now, studies addressing the investigation of EVs are still showing a poor combination of different methodologies exploring complementary aspects. Thus, even if several EV features have been addressed, a complete comprehension of the mechanisms regulating EVs’ genesis, spreading, and functionality remain unclear in most of the studies, where only a partial view is presented. To fill this gap and provide a holistic view of the features of EVs, we believe in the need to integrate the various available techniques and methodologies. Besides improving the understanding of the features of EVs, such combinations may represent a boost to unravel the complex network of relationships among EVs, their origin, and the fate of their target cells. Thus, the goal of the present review is to emphasize the complementarity of experimental approaches and studies in which authors integrated them strategically to gain a comprehensive understanding of the different aspects playing a role in EVs’ properties and functionality.
2. Analytical and Technical Aspects in EVs
2.1. Isolation, Purification, and Preparation
Due to the high heterogeneity of EV populations, sample purity is a key premise for reliable outcomes, especially in the context of multiple data integration. Despite the recent efforts in this field, the isolation, purification, and preparation methodologies are not yet fully mature and more in-depth studies are still required to improve EV subtyping. Moreover, EVs are often isolated from body fluids (urine, serum, plasma, saliva, tears, breast milk, gastric juice, pleural effusions, malignant effusions, as well as in prostatic, amniotic, cerebrospinal, synovial fluids, and bronchoalveolar lavage fluids) which represent a source of contaminants, usually several orders of magnitude higher than that of EV components, thus interfering with the downstream analysis.20 It is important to highlight that isolation/purification methods should take into account the sample source, volume, purity, integrity, subsequent analyses, instrumentation, and processing time. In this context, it should also be considered that the different methods could affect final EV composition, and this represents a relevant aspect when different studies are evaluated, compared, and integrated.1
Several current protocols for the isolation of EVs are mainly based on methods as ultracentrifugation (UC), density gradient purification, polyethylene glycol (PEG) precipitation, tangential flow filtration (TFF), and size exclusion chromatography (SEC). Every method has its own pros and cons on EV purity and may not be generally suitable for diverse experimental purposes.20 For example, UC, which represents the gold standard to isolate EVs, does not separate them from free ribonucleoproteins and lipoproteins, distorting RNA data. Thus, some authors proposed SEC with a density cushion, whereas others used high-resolution density gradient fractionation coupled to immunoaffinity capture. Similarly, isolation protocols are fundamental for proteomic analysis. Indeed, often, EVs are isolated from biological fluids where the high dynamic range of the expressed proteins may interfere with the effective EVs’ proteome characterization. As a consequence, the correlation of biophysical and biochemical properties of isolated EV subpopulations with their biological function is under continuous debate, due to the difficulty in establishing an accepted standard for isolation processes.20 On the other hand, a multifaceted characterization can allow an “ID card” of the different cell-derived EVs to be obtained. Of note, this strategy recently led to the discovery of two new families of very small EVs: exomeres,3 defined as nonmembranous nanovesicles with a size of ≤50 nm, and supermeres,2 even smaller particles highly enriched with cargo molecules.
If specific EVs often need to be isolated from the originating sample to perform molecular/structural/morphological analysis on purified samples, sometimes their detection in a complex sample is enough for diagnostic purposes to identify biomarkers related to specific diseases.
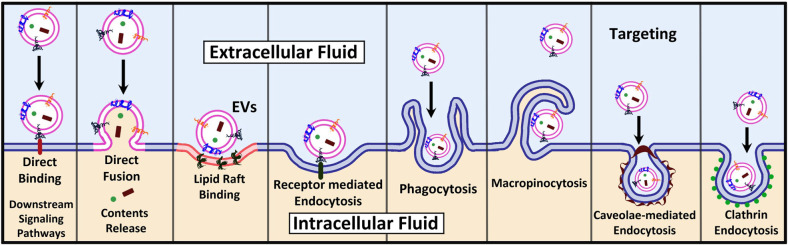
In this context, among the variety of the existing internalization mechanisms7 of EVs (Figure 4), understanding the exact one through which each specific EV interacts and is internalized into its target cell would be beneficial for the development of accurate detection techniques, exploiting the unveiled specific interaction to detect and eventually isolate the EV of interest. Such an approach would be of great importance, for example, when a specific EV is being investigated and searched for in a multicomponent complex environment, such as body fluids.
Figure 4.
EV internalization mechanisms are different and depend on the specific EVs and the considered target cell/tissue.
2.2. Structure of Vesicular Aggregates as a Whole at the Mesoscale
Following the EVs’ isolation, the knowledge of their size distribution is of great importance because it reflects different uptake kinetics and biological functions used to classify them. The use of complementary techniques for the size characterization of EVs is of crucial importance for their applications in diagnosis and therapy.8 Indeed, different techniques could report different size values since they rely on different physical parameters and instrument settings. Due to the wide availability of user-friendly instruments, LS is among the techniques mostly used to measure the size distribution of the objects in solution; even if in LS measurements the scattering volume is big, and the correlation function accounts for all of the particles altogether. In this respect, nanoparticle tracking analysis (NTA) is more suitable for describing the size distribution of EVs, allowing the particles to be counted one by one. Nonetheless, in comparison to NTA, LS remains a useful technique, detecting particles sized less than 50 nm, with an advantage with respect to other complementary techniques of performing noninvasive measurements without implying any modification of the shape of the objects under investigation. On the other side, microscopies also access the EVs’ morphological features. For this purpose, electron microscopy (EM) and AFM represent the gold standard methods. In particular, cryo-EM allows the presence of a lipid bilayer to be demonstrated, but it is still not an easily accessible instrumentation even though its use is increased after the Nobel Prize in 2017. On the contrary, transmission electron microscopy (TEM) is more accessible than cryo-EM, but it has the drawback of sample drying, fixation, and staining with uranyl acetate, with all of the related issues of drying effects on the final evaluation of dimensions (drying and fixation) and safety issues (uranyl acetate). Nevertheless, both cryo-EM and TEM allow sub-nanometer resolution, providing details not accessible by other techniques. On the other hand, AFM presents the advantage of working in liquid conditions, mimicking the natural EV microenvironment. Due to its versatility, AFM has been shown to be extremely useful in verifying size, morphology, mechanical properties, distribution of markers, surface potential, and surface roughness. It allows the subpopulations of EVs to be distinguished and highlights morphological and/or mechanical changes among EVs derived from different cell lines or isolated through different methodologies.9,20 In this scenario, X-ray and neutron scattering techniques add important information about EVs’ structural properties and mean molecular composition,10 otherwise impossible to achieve with a less than nanometer resolution in a noninvasive way. Specifically, neutron-based techniques, such as small-angle neutron scattering (SANS) and neutron reflectometry (NR), are less destructive than X-ray-based ones even if their limiting factor is a smaller accessible q range and a high background coming from the hydrogen.
2.3. Basic Constituents and Cargo Profiling
In addition to basic structural constituents, as carriers of biomolecules, EVs are able to transfer DNA, RNA species, proteins, lipids, and metabolites to target cells, in both local and distal environments.5 These extensive heterogeneous data represent a valuable source of information suitable in performing multi-biomarker discovery for diagnostic and EV subtyping purposes.2,3,7 Moreover, their knowledge could help to shed light on the biogenesis mechanisms of EVs as well as on pathophysiological processes mediated by EVs’ signaling messages. It is well-known that soluble molecules, such as growth factors, cytokines, chemokines, hormones, and neurotransmitters, play key roles in intercellular communication. Achieving these goals correlates with the important challenges that EVs’ genomics, proteomics, and metabolomics must address, including small size, broad dispersion in dimensions, cargo, membrane composition, biogenesis, and the extremely heterogeneous space of biological functions. However, to date, there is a set of advanced analytical omics technologies available to characterize molecular profiles of biological samples, representing a good starting point to classify all molecules composing EVs.
For basic structural constituents, such as proteins and lipids, significant information could be retrieved using Fourier transform infrared (FTIR) spectroscopy and Raman spectroscopy. These techniques allow the pattern of covalent bonds to be studied by analyzing the infrared (IR) absorptions due to vibrational modes of constituent molecules and molecular functional groups. Intensity, position, and width absorption bands give information on the chemical composition of a sample and the relative concentration of its different constituents. In particular, FTIR spectra can be used to evaluate the protein/lipid ratio. The analysis of FTIR bands also provides information about protein orientation and whether the form of protein is helical, sheet, or random. FTIR spectra can also differentiate among different EV subpopulations, collectively fingerprinting them in one single experiment. Similar information can be retrieved by Raman spectroscopy, with the advantage of performing measurements in a water environment, whereas FTIR suffers from the high water absorbance and needs dehydrated or dried samples. However, instrument advances for using FTIR in aqueous environments were recently developed to overcome that obstacle.
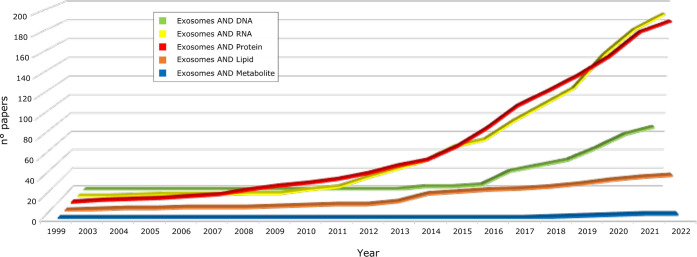
To investigate deeply the EVs’ molecular constituents, spectroscopic techniques must be combined and complemented by approaches allowing a detailed molecular profiling of DNA, RNA, proteins, lipids, and metabolites. Due to the constant development and improvement of high-throughput omics technologies, mainly NGS and MS-based proteomics, lipidomics, and metabolomics in the last years, many efforts have focused on EVs’ molecular characterization.2,3 Taking as a reference the most investigated EV type, exosomes, it emerges that most studies focused on shedding light on proteins and RNA species, whereas less studies have been dedicated to DNA and lipids and even less to metabolites, as summarized in Figure 5.
Figure 5.
Cumulative number of papers focusing on exosome omics profiling from 1999 to April 2022 (collected from www.pubmed.ncbi.nlm.nih.gov); papers were selected by the presence of “exosomes” AND different classes of molecules in their title.
For DNA, only recently has the evidence emerged that EVs contain single-stranded DNA (ssDNA), double-stranded DNA (dsDNA), and mitochondrial DNA. In particular, exomeres and chromatimeres have been championed as potential carriers of large amounts of cell-free DNA (cfDNA), yet their function remains poorly understood.3 In fact, in comparison to proteins and RNAs, whole-genome sequencing was recently used to investigate EV-transferred DNA species with few studies available to date.21 Nevertheless, extracellular cfDNA is attracting interest from the scientific community due to its potential as a biomarker, mainly in cancers, as well as its putative implication in regulating signaling pathways and pathophysiological processes. On the other hand, extracellular DNA release can occur through apoptotic and necrotic cell death. For this reason, major efforts are required to establish DNA origins and properties. The achievement of this goal is currently pushing the development of appropriate protocols to discriminate EVs’ DNA origin from passive product of DNA fragmentation. For example, a major topic concerns which EV populations are actually associated with DNA. Moreover, EV DNA packaging (both genomic and mitochondrial), the correlation between DNA structure and pathophysiological states, and its function in recipient cells represent some of the crucial topics that need to be addressed in forthcoming future studies to broaden and consolidate our knowledge of EVs as a whole.
Unlike DNA, in the last 10 years, a special interest has been directed to the variety of existing RNA molecules (micro-RNAs (miRNA), messenger RNAs (mRNA), ribosomal RNA (rRNA), transfer RNA (tRNA), small nucleolar RNA (snoRNA), small nuclear (snRNA), long noncoding RNA (lncRNA), long intergenic noncoding RNA (lincRNA), and noncoding RNA (ncRNA)) carried by EVs.14 RNAs are internalized by target cells, where, in cooperation with other molecules, they affect gene regulatory networks and thus the transcriptional and translational processes. However, the functional role of the RNA cargo seems mainly driven by miRNAs and lncRNAs, whereas the role of others, including tRNAs, mRNA fragments, or rRNA, remains unclear. Several studies correlated the horizontal transfer of EV miRNA cargo with controlled tumor growth, affecting the microenvironment and promoting metastases by reprogramming of recipient cells.2 In addition, it has also been proved that the EVs’ miRNAs and lncRNAs, have a role in tumor drug resistance,2 cardioprotection,6 and neurogenesis, as well as in regulating gene expression, along with affecting obesity or diabetes.14 The most popular method to characterize RNA molecules is the quantitative reverse transcriptase PCR (qRT-PCR). It is simple and low-cost, requires a small amount of sample, and does not require bioinformatics skills. However, its major drawback concerns the limited heterogeneity of RNA populations that may be explored as it is primarily used to measure the amount of specific RNAs following a proper design of primers that target the sequence of interest, thus it does not fit with gene expression measurements on a global scale. On the contrary, NGS has been proven to be a method for a comprehensive analysis of the RNA families.
Similar to RNAs, in the past decade, EVs have been the subjects of several proteomic studies aimed at deepening their isolation, analysis and characterization. Among EVs’ transported molecules, proteins represent the main effectors of biological functions and, together with miRNA, directly regulate the transcriptional program of recipient cells. Their knowledge and correlation are therefore fundamental for understanding EVs’ function, biogenesis, and discovery of markers for diagnostic purposes,6 as well as EV subtype discrimination since protein cargo of EVs is both cell- and disease-type-dependent.15 In order to perform EV subtype discrimination, the most popular approaches rely on the combination of biophysical findings and the measurement of common markers, including CD63, CD9, and CD81. On the contrary, the EVs’ proteome cargo is investigated by high-throughput analytical approaches. Although EVs’ proteomic investigations have also been successfully performed by gel-based approaches, the increasing progress in both liquid chromatography (LC) and high-resolution MS has driven more and more the use of gel-free methodologies.16 They proved their effectiveness for qualitative and quantitative proteome profiling of EVs, as well as for the identification of post-translational modifications (PTMs). Providing information on the activation status of various proteins may affect protein–protein interactions, as well as their targeting to EVs.1
The main MS-based approaches used for proteomics studies, including for EVs, are categorized as discovery and targeted proteomics to address relative and absolute quantification (by both label and label-free strategies). However, while discovery proteomics is usually applied for protein profiling and quantitative analysis, targeted quantitative proteomics, as selective reaction monitoring (SRM) and sequential window acquisition of all theoretical fragment ion spectra mass spectrometry (SWATH-MS), aim at validating protein expression of a preselected group of proteins/peptides.16 All of these approaches, combined with complementary biophysical findings, are useful for improving the characterization of EV subtypes as well as for correlating them to pathophysiological states. To reach this goal, major limitations in using proteomics of EVs are still related to their heterogeneity even after isolation. In fact, it has been observed that highly purified EV subtypes from the same cellular origin are biochemically and functionally distinct. Thus, also in this case, the combination of complementary strategies to classify homogeneous EV subpopulations represents the proper approach for improving the correlation between chemicophysical, molecular, and clinical aspects.2,3,6,16,17 In addition, such an integrative strategy is crucial to extract reliable markers for effective monitoring of EVs’ isolation, their subtyping, and more importantly for developing diagnostic tools.
Unlike RNAs and proteins, much less information is available about the content of cargo in terms of metabolites. However, EVs are already recognized as independent metabolic units that can modulate systemic changes in recipient cells. Experimental hardships represent the major limitation in studying the EVs’ metabolomics;17 in fact, EVs’ cargo includes sub-nanomolar concentrations of small metabolites (carbohydrates, amino acids, nucleotides, enzymatic cofactors, and lipids) whose enrichment is preferable prior to spectrometry assays. In this scenario, lipids are one of the most structurally heterogeneous classes of biomolecules present in EVs due to their permutations in head groups and fatty acid chains. If from one side lipids have a role in EV structure and stability, they are also bioactive mediators.22 It has been shown that different classes of lipids are present in EVs, whose cargo is enriched in lipids such as glycerophospatidylcholines and glycerophospatidylethanolamines. Eicosanoids have been associated with EVs involved in inflammatory processes; it is well-established that eicosanoids are implicated in multiple diseases, including asthma and cancer, or biological processes, such as hemostasis, immunity, and reproduction. For example, regarding breast cancer, some studies correlated a different lipid composition in EVs from breast cancer cell lines, as well as tumor cells with low metastatic potential versus high metastatic potential.22 At an analytical level, MS-lipidomics allow high sensitivity, specificity rates, and an accurate measurement of the molecular weight of the analyzed lipids. However, overlaps in different classes of lipids are still posing great challenges in exact identifications. More recently, thin layer chromatography (TLC) coupled with mass spectrometry systems such as matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) has also been described as an efficient method in discriminating the lipidomic content of EVs. Notably, this approach has been used not only to identify but also to differentiate the lipid composition of EVs’ subpopulations.
A specification must be done to clarify that the above-mentioned omics approaches are not usually applied to distinguish the molecules embedded into the EVs’ membranes from those transported into the EVs’ core, unless a subfractionation or a targeting analysis is performed. As an example, recently, Xu et al.23 focused their attention on exosome surface-exposed membrane proteins (surfaceome) after their proteolytic shaving. An estimation of the relative amounts of lipids and proteins embedded or not in EV membranes can also be performed by techniques such as FTIR or NR by comparing the results obtained by studying dry samples (where all molecules from solution are deposited) and EVs derived from single supported membranes in water environment (where only the EV’s membrane is fused).
3. Case Studies: From Biophysics to Profiling Integration
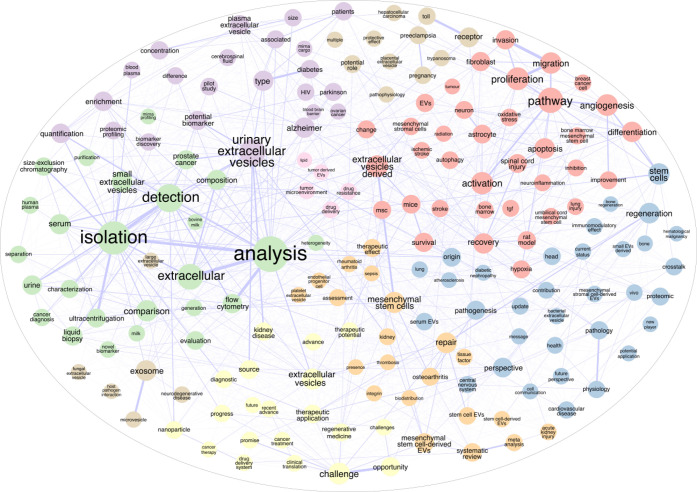
The increase of studies on EVs that we are observing in recent years is spurring the need for new techniques and devices that can surmount experimental roadblocks, as detailed in the previous sections. As already established, the urgent need for scientific research performed on EVs, linking their importance to different physiological and pathological effects, triggered the emergence of thousands of research articles aiming at their characterization as well as diagnostic and therapeutic investigation approaches, as schematized in Figure 6.
Figure 6.
Word association from manuscript titles containing “extracellular vesicles” OR “EVs” showing the most significant fields currently under study in the ongoing research of EVs collected from www.pubmed.ncbi.nlm.nih.gov (number of papers = 6909).
Following what is reported in the Introduction, and in line with this review’s topic, in this section, we will introduce some examples where different experimental approaches were integrated with the purpose to exploit their complementarities and to provide a holistic view of the investigated EV systems.
Based on these premises, two recent studies allowed the discovery of new EV families: exomeres3 and supermeres.2 In the first study,3 published in 2018, Zhang et al. employed the asymmetric-flow field-flow fractionation (AF4) to resolve two exosome subpopulations (Exo-L, Exo-S) and to discover an abundant population of nonmembranous nanoparticles called “exomeres” (∼35 nm). By integrating data from different omics approaches, the authors found that Exo-S, Exo-L, and exomeres had distinct N-glycosylation, protein, lipid, DNA and RNA profiles, and biophysical properties. Specifically, exomeres were enriched in proteins involved in metabolism, mainly glycolysis and mTORC1 pathways, as well as in proteins associated with coagulation and hypoxia. Moreover, they were enriched in proteins controlling glycan-mediated protein folding control (CALR) and glycan processing (MAN2A1, HEXB, GANAB), suggesting a role in modulating glycosylation in distant targeted cells. In agreement with structural studies that suggested exomeres as non-encapsulated particles, membrane-associated proteins were poorly represented. On the contrary, Hsp90-b was highly present in exomeres, playing a role as a potential marker. For this purpose, glycomic, lipidomic, and genomic analyses provided further sources of data to select additional molecular signatures. For instance, high levels of triglycerides and ceramides were found in exomeres, whereas DNA analysis revealed that DNA packaging was correlated with tumor-type ones.3
In a more recent study,2 Zhang et al. discovered another class of extracellular nanoparticles, called “supermeres”, morphologically distinct from exomeres. By combining sequential high-speed ultracentrifugation protocols, fluid-phase AFM and TEM imaging, they revealed that the morphological structure of supermeres was distinct from small EVs (sEVs), nonvesicular (NV) nanoparticles, and exomeres derived from two human colorectal cancer (CRC) cell lines, DiFi and HCA-7-derived spiky colony, and from the human breast cancer cell line MDA-MB-231. Using liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS), the authors pointed their attention to the supermeres’ protein content, finding that it was enriched in proteins related to metabolism. In addition to enzymes involved in fatty-acid metabolism, enrichment analysis of differentially expressed proteins (DEPs) showed that many enzymes involved in glycolysis were highly enriched in supermeres compared to sEVs and exomeres. In particular, enolase2 (ENO2) was highly associated with supermeres. This marked enrichment of glycolytic enzymes drove the authors to examine if and how supermeres could alter the metabolism of recipient cells. They demonstrated that the addition of cancer-cell-derived supermeres to recipient cells increases lactate secretion and is able to transfer cetuximab resistance and decrease hepatic lipids and glycogen in vivo. At the protein level, supermeres also showed high levels of TGFBI in DiFi supermeres and the second-most abundant in PANC-1 supermeres, whereas the glycolytic enzyme enolase1 (ENO1) was the most abundant in the PANC-1 and MDA-MB-231 supermeres. However, the heat shock protein HSPA13 was enriched in the supermeres of all analyzed cell lines (DiFi, PANC-1, MDA-MB-231, SC, and human renal epithelial (HREC)), suggesting a potential role as a protein marker. In addition to the proteome, the authors found a distinctive supermere small extracellular RNA list with a relatively high percentage of snRNAs. In this scenario, exomeres and supermeres showed miRNA expression patterns closely related but distinct from that of sEVs and specific cells. The most highly abundant and enriched miRNAs in exomeres included miR-92a-3p, miR-1247-5p, and miR-10a-5p, and the authors validated the expression of supermere-enriched miR-1246 and miR-675, further revealing signatures for this new class of EVs. As a completion of this multimodal study, the authors investigated the uptake dynamics in vitro of sEVs, exomeres, and supermeres derived from DiFi cells in treated MDA-MB-231 cells. They found that supermeres were efficiently taken up by multiple organs, including the liver, lung, colon, heart, and brain. Thus, as functional agents of intercellular communication, they represent candidate biomarkers and therapeutic targets in several disease states including cancer, cardiovascular disease, Alzheimer’s disease, and SARS-CoV-2 infection.
Another example of fruitful analytical integration comes from Wong’s group in the field of cancer biomarkers. Wong and colleagues performed a variety of investigations complementing biochemical and biophysical aspects to obtain get a comprehensive description of exosomes from salivary glands, proposed as cancer biomarkers.12 Immunoblotting, flow cytometry, and fluorescence-activated cell sorting (FACS) analysis showed the enrichment of the vesicles with CD63, while the presence of 509 core mRNA transcripts was monitored through microarray analysis. The transfer of mRNAs, from the salivary EVs to the human oral keratinocytes, was observed using labeled vesicles with fluorescent lipid BODIPY-PC. The proteomic analysis proved the expression of annexin A1, annexin A2, moesin, and OS-9 proteins, and it suggested the influence of salivary EVs on oral keratinocytes. qRT-PCR was also used to investigate the presence of specific modulated transcripts in their cargo. Recently,9 authors investigated their substructure using ultrasensitive low-force AFM, correlated with field emission scanning electron microscopy (FESEM), to interpret the exosomes’ nanoscale structures under varying forces. EVs, sized around 70–100 nm and showing trilobed morphology, were observed to show a reversible mechanical deformation displaying distinct elastic properties. Force spectroscopy with anti-CD63 IgG-functionalized AFM tips proved the detection of the antigen CD63, an important cancer biomarker, on their surface. Moreover, by implanting mice with human lung cancer H460 cells expressing hCD63-GFP, the authors monitored the circulating tumor cells in vivo. Finally, electric-field-induced release and measurement (EFIRM) along with qRT-PCR could monitor human GAPDH mRNA in salivary EVs in the tumor-bearing mice, proving the possibility to exploit biomarkers in the salivary EVs for biomedical applications.18
Moving toward the integration of structural, biochemical, and biophysical techniques to highlight the specific behavior of EVs, it is worth citing the work of Romancino et al.24 as a multidisciplinary effort to investigate the effects of palmitoylation on the membrane organization of small extracellular vesicles. The fruitful combination of bioinformatics tools, biochemical assays, Western blots, AFM, small-angle X-ray scattering (SAXS), and SANS allowed the biological function of S-palmitoylation in the regulation of Alix proteins to be understood. Palmitoylation facilitates the interactions among EV-specific regulators as CD9 and helps in maintaining the proper structural organization of the membranes. In particular, they were able to distinguish the effects of chemical inhibition of palmitoylation on small (sEV) and large (lEV) extracellular vesicles. Inhibition of palmitoylation mainly influences the sEV population with an induced larger secretion as monitored by AFM, DLS, and NTA analyses and with a modification of lipid bilayer organization as revealed by SAXS and SANS data. Beyond the biological relevance, this work was the first to exploit neutron scattering techniques for the analysis of extracellular vesicles.
Such techniques have been recently exploited to shed new light on the mechanisms by which EVs recognize and bind to specific cells in the case of mesenchymal stem cell derived EVs for therapeutic applications. Indeed, addressing the mechanisms of fusion and release of cargo is necessary not only to understand but also to mimic and engineer new delivery strategies. Building up on the work done by Gimona’s group on potency assays for mesenchymal stem cell derived EVs,25 Perissinotto and Rondelli et al.10 applied a set of complementary techniques to unveil new aspects of mesenchymal stem cell derived EV characteristics and internalization mechanisms. After the chemicophysical characteristics of EVs by NTA, cryo-EM, and multiplexed surface marker analysis, a combination of SAXS, SANS, and NR were used to assess the membrane bilayer structure and lipid/protein ratio (by volume) of sEV membranes. Specifically, the lipid/protein ratio of the EV membranes was obtained by NR from EV-derived supported bilayers, which was the contrast to neutrons defined by the sum of the different molecule contributions. On the same paper, the authors also investigated EVs’ fusion mechanisms, where a combination of AFM, NR, and SANS allowed the details of sEVs’ internalization into synthetic lipid membranes containing raft domains to be described. This joint exploration allowed them to observe that EVs interacting with artificial lipid bilayers break and form EV–membrane domains, whose areas increase over time, suggesting the formation of initial nucleation seeds which act as docking sites for other EVs from solution. The lipid phase borders present on the artificial bilayers (raft-like structures) seem to act as preferential docking sites from where the different sEV components spread with different kinetics in the surrounding bilayer. Further insights on the fusion mechanism of such systems have been explored by the same groups by means of differential scanning calorimetry and dynamic light scattering,26 and these experiments allowed them to observe after fusion of sEVs with artificial lipid vesicles (LUVs) an increased rigidity of the bilayer, a flattening of the membrane with an increase in size of the LUVs, a decrease in the lateral mobility of the lipids, and a decreased transition cooperativity, as a sign of an increased membrane parcellation. Such experiments are important not only because they shed light on the likelihood of EVs to interact with specific domains with respect to others but also because they allowed the investigation of the effects of EVs on target cell membranes, opening perspectives in this field that are applicable to EVs of different origin.
With a similar perspective, Montis et al.11 combined different experimental techniques to investigate the interaction of superparamagnetic iron oxide nanoparticles (SPIONs) with supported lipid bilayers, synthetic and derived from EVs extracted from prostatic tumors (from the murine cell line TRAMP-C2). Several complementary techniques, such as quartz crystal microbalance (QCM), X-ray reflectivity, grazing-incidence small-angle X-ray scattering (GISAXS), AFM, and confocal microscopy, were used to perform this study. The authors could prove that the adhesion of SPIONs on the lipid membranes was strongly encouraged for EV-derived supported lipid bilayers, possibly due to their higher compositional complexity with respect to the synthetic ones, which leads to a significant enhancement in the response to the adhesion of nano-objects. In addition, they could observe that supported membrane features are changing in response to SPION interactions. The successful use of such complementary physical techniques in this study could strengthen the understanding of the structural and physicochemical features of the EV-derived supported lipid bilayers, thus leading to a significant view of their possible manipulation as 2D platforms for biosensor applications.
4. Discussion and Conclusions
Based on our recent literature searches, a real integration of diverse techniques aiming at investigating the complementary aspects characterizing EVs, in terms of structure, morphology, molecular cargo, and function, has been rarely performed. Although it usually happens when different groups with specific expertise carry out complementary investigations on the same EV systems, highlighting the importance of merging their findings, few are the cases where in the same study the authors presented the investigation of EVs in all their aspects.2,3,10,12 Due to the orthogonality of the different fields, requiring complementary expertise, instruments, computational tools, as well as funds, the snapshot of the current situation is not surprising. However, the case studies we have put under the magnifying lens in this review unveiled how integration strategies are productive in terms of results and findings. As emerged by our in-depth analysis, a comprehensive strategy was demonstrated to be effective in discovering new EV classes,2,3 as well as in describing EV-specific internalization mechanisms.10,13 The use of complementary techniques strengthened the understanding on the structural and physicochemical features of the EV-derived supported lipid bilayers,10,11 which are of interest not only for their characterization but also for their beneficial manipulation and exploitation as platforms. The application of structural techniques,24 linked to molecular profiling, allowed researchers to investigate the direct effect of changes in cells as inhibition of palmitoylation on cell–cell communication by affecting the secreted EV population.
In this scenario, moreover, the multiomics approaches adopted, associated with biophysical techniques, led to the discovery of multiple and diverse markers which represent a valuable source of information to be exploited for diagnostic, prognostic, and therapeutic purposes.6,9,12 Indeed, it is not a coincidence that most of the case studies presented here, as well as other studies cited in our review, focused on tumor investigation.2,3,8,11,12,16−18,23 In this field, in fact, the EVs’ role in cellular communication has often been associated with the favoring of the tumor microenvironment, as well as with the induction of drug resistance, thus representing potential therapeutic targets of interest.
Although the correlation of multiple omics data reflecting the EVs’ cargo content represents a key aspect for extracting distinctive signatures,2,3 as well as to predict the effect of EVs on target cells,6,27 their integration with other types of EV data and findings can improve the understanding of the relationships among structures, cargos, and functions mediated by EVs. The need for data integration originates from the holistic view of the cell’s response to different conditions, and data-derived system biology approaches could represent a powerful strategy for a comprehensive understanding of the EVs’ complexity and their role in cellular communication.1,6,27,19 The major challenges in evaluating EVs as biological systems concern their high dimensionality (many players involved) and connectivity (many connections between players). To address these difficulties, as for omics profiles, holistic methods based on network analysis represent a tool for associating heterogeneous data and unraveling the tangle of molecular relationships in which EVs may be involved. These methods can be used to assess modulatory relationships among omics layers. In this scenario, it has already been reported that vesicular molecules (RNAs, proteins, lipids, and metabolites) are physically interconnected into functional modules involved in several and well-defined biological processes, including vesicle-mediated transport, cytoskeletal organization and immune systems.1,19 However, integrating transcriptomics data with proteomics and metabolomics is per se a challenge in cell lines or tissues due to their low correlation. Rather, a more attractive strategy should rely on the correlation between EVs’ structure/cargo molecules with the effect predicted and observed in target cells, as showed in some studies were authors combined the EVs omics profiles experimentally defined, with network models (including protein–protein interactions (PPIs), miRNA targets) reconstructed from knowledge accumulated and stored in specific repositories.1,6,27,19 Noteworthy, in this context, novel approaches put an emphasis on the topology of the reconstructed network models with the purpose to detect pathways affected by the treatment of EVs and hubs as molecules targeted by EVs.6,27
In the future, we expect that new techniques will be developed or adapted to investigate EVs in order to get faster and more accurate findings. Regardless, with the employed technologies, relevant efforts must be focused on improving the isolation and purification methods20 which represent an essential prerequisite for accuracy. With the same purpose, specific efforts still have to be made concerning the standardization of sample collection and processing. It is also fundamental to obtain reproducible data sets to be compared for integrative purposes. Improved reporting of experimental design and results is as well an additional aspect potentially affecting reproducibility. Moreover, the availability and consistency of previously reported data are major limitations for validation and meta-analysis studies. Although data heterogeneity can be substantially induced by the different protocols applied, large collections of data can yield more statistically significant observations. In this scenario, the multidisciplinary interest in investigating EVs has driven the development of databases that store experimental transcriptomics, proteomics, metabolomics, and lipidomics data sets, as well as isolation, preparation, and characterization protocols. Some of them, such as Vesiclepedia (http://www.microvesicles.org/) and ExoCarta (http://exocarta.org/) are specifically dedicated to EVs, whereas others, such as EVmiRNA (http://bioinfo.life.hust.edu.cn/EVmiRNA), are related to specific EV molecules. In addition, for proteomics, repositories like MassIVE (https://massive.ucsd.edu/ProteoSAFe/static/massive.jsp), PRIDE (https://www.ebi.ac.uk/pride/), and PeptideAtlas (http://www.peptideatlas.org) could represent a further source of experimental data on EVs, whereas GEO (https://www.ncbi.nlm.nih.gov/geo/) serves the same function for genomic and transcriptomic profiles. On the other hand, plenty of resources (www.pathguide.org) are dedicated to network models, including protein–protein interactions, metabolic pathways, signaling pathways, and gene regulatory networks, representing key information for understanding the complex relationships that govern cellular communication. Such stored information and their continued accumulation and improvement in accuracy represent an opportunity for researchers in connecting previous findings, designing their studies, and driving them to a comprehensive holistic view of the EVs considered. Undoubtedly, new avenues in diagnostic, prognostic, and therapeutic applications of EVs will be opened by following these integrative strategies, combining biophysical approaches, high-throughput omics technologies, and models taking into account the players involved in cellular communication, including EVs, targets, and mediators.
Acknowledgments
S.H. is funded by a full scholarship (mission 2019/2020) from the Ministry of Higher Education in Egypt. The current work is not founded by the mentioned ministry of Egypt.
Glossary
Abbreviations
- AF4
asymmetric-flow field-flow fractionation
- AFM
atomic force microscopy
- cfDNA
cell-free DNA
- CRC
colorectal cancer
- Cryo-EM
cryogenic electron microscopy
- DEPs
differentially expressed proteins
- DLS
dynamic light scattering
- dsDNA
double-stranded DNA
- EFIRM
electric field-induced release and measurement
- EM
electron microscopy
- EVs
extracellular vesicles
- FACS
fluorescence-activated cell sorting
- FESEM
field emission scanning electron microscopy
- FTIR
Fourier transform infrared spectroscopy
- GISAXS
grazing-incidence small-angle X-ray scattering
- HREC
human renal epithelial cancer
- IR
infrared
- LC
liquid chromatography
- LC-MS/MS
liquid chromatography coupled to tandem mass spectrometry
- lincRNA
long intergenic noncoding RNA
- lncRNA
long noncoding RNA
- LS
light scattering
- MALDI-TOF
matrix-assisted laser desorption ionization time of flight
- miRNA
microRNAs
- mRNA
messenger RNAs
- MS
mass spectrometry
- MVB
multivesicular bodies
- MVs
microvesicles
- ncRNA
noncoding RNA
- NGS
next generation sequencing
- NR
neutron reflectometry
- NTA
nanoparticle tracking analysis
- NV
nonvesicular
- PCR
polymerase chain reaction
- PEG
polyethylene glycol
- PTMs
post-translational modifications
- QCM
quartz crystal microbalance
- qRT-PCR
quantitative reverse transcriptase PCR
- rRNA
ribosomal RNA
- SANS
small-angle neutron scattering
- SAXS
small-angle X-ray scattering
- SEC
size exclusion chromatography
- sEVs
small EVs
- snoRNA
small nucleolar RNA
- snRNA
small nuclear RNA
- SPIONs
super-paramagnetic iron oxide nanoparticles
- SRM
selective reaction monitoring
- ssDNA
single-stranded DNA
- SWATH-MS
sequential window acquisition of all theoretical fragment ion spectra mass spectrometry
- TEM
transmission electron microscopy
- TFF
tangential flow filtration
- TLC
thin layer chromatography
- tRNA
transfer RNA
- UC
ultracentrifugation
Biographies
Valeria Rondelli is a Researcher in Applied Physics in the Department of Medical Biotechnologies and Translational Medicine of the Università degli Studi in Milano, Italy. After receiving her Master’s degree in Physics in 2008, she received her Ph.D. in Biochemistry in 2012 from the Università degli Studi in Milano. Her research focuses on the structural investigation of biomolecules and bioaggregates in solution and at interfaces with a special focus on biological and biomimetic membranes, by means of experimental techniques including the scattering and reflectometry of laser light, neutrons and X-rays, Langmuir films and depositions, DSC, and QCM-D.
Sally Helmy is a Ph.D. student in Applied Physics in the Department of Medical Biotechnology and Translational Medicine in the Università degli Studi in Milano, Italy. After receiving her Master’s degree in Biophysics in 2016 from the Faculty of Science, Ain Shams University, Egypt, she worked there as an assistant lecturer and then she received a full Ph.D. scholarship from the Ministry of Higher Education in Egypt to pursue her Ph.D. study in Università degli Studi in Milano, Italy. Her current research focuses on the structural investigation of biomolecules and nanovesicles as EVs in solution and at interfaces focusing on biomembranes interactions, by means of diverse biophysical techniques such as scattering and reflectometry of laser light and neutrons, DSC, FTIR, AFM, and QCM-D.
Giulia Passignani is a research fellow at the Institute for Biomedical Technologies of Italian National Research Council (ITB-CNR) in Segrate (Milan). She received a Master’s degree in Molecular Biotechnology and Bioinformatics at Università degli Studi in Milano, Italy, in 2020. Her research focuses on omics data analysis, mainly proteomics and transcriptomics, by a computational and programming activity aimed at processing data and developing algorithms.
Pietro Parisse is a Permanent Researcher at the Istituto Officina dei Materiali of Italian National Research Council in Trieste. He received his Ph.D. from University of L’Aquila in Italy (2009), followed by postdoctoral fellowships and junior researcher positions at Elettra Synchrotron Radiation facility in Trieste (2009–2020). His research focuses on the study of structure and functionality of biomolecules (DNA, RNA, proteins) and nanovesicles (e.g., EVs) by means of scanning probe microscopies and nanobiophysical tools.
Dario Di Silvestre is a Permanent Researcher at the Institute for Biomedical Technologies of Italian National Research Council (ITB-CNR) in Segrate (Milan). He received his degree in Agricultural Biotechnology from University of Milano (Italy) in 2004 and his second level Master’s degree in Bioinformatics from University of Milano-Bicocca (Italy) in 2006. His research focuses on the functional characterization of omics profiles, mainly proteomics and transcriptomics, using system biology approaches based on graph theory, including protein–protein interaction (PPI) and coexpression network models. The research fields explored by these approaches include basic research and human diseases, as well as plant organisms.
Author Contributions
# P.P. and D.DS. contributed equally to this paper.
The authors declare no competing financial interest.
References
- Chitoiu L.; Dobranici A.; Gherghiceanu M.; Dinescu S.; Costache M. Multi-Omics Data Integration in Extracellular Vesicle Biology—Utopia or Future Reality?. Int. J. Mol. Sci. 2020, 21 (22), 8550. 10.3390/ijms21228550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Jeppesen D. K.; Higginbotham J. N.; Graves-Deal R.; Trinh V. Q.; Ramirez M. A.; Sohn Y.; Neininger A. C.; Taneja N.; McKinley E. T.; et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nat. Cell Biol. 2021, 23 (12), 1240–1254. 10.1038/s41556-021-00805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Freitas D.; Kim H. S.; Fabijanic K.; Li Z.; Chen H.; Mark M. T.; Molina H.; Martin A. B.; Bojmar L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20 (3), 332–343. 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch Y.; Buzàs E. I.; Di Vizio D.; Gho Y. S.; Harrison P.; Hill A. F.; Lötvall J.; Raposo G.; Stahl P. D.; Théry C.; et al. A brief history of nearly EV-erything–The rise and rise of extracellular vesicles. J. Extracell. Vesicles 2021, 10 (14), e12144. 10.1002/jev2.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkach M.; Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell 2016, 164 (6), 1226–1232. 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- Burrello J.; Tetti M.; Forestiero V.; Biemmi V.; Bolis S.; Pomatto M. A. C.; Amongero M.; Di Silvestre D.; Mauri P.; Vassalli G.; et al. Characterization of circulating extracellular vesicle surface antigens in patients with primary aldosteronism. Hypertension 2021, 78 (3), 726–737. 10.1161/HYPERTENSIONAHA.121.17136. [DOI] [PubMed] [Google Scholar]
- Pachler K.; Ketterl N.; Desgeorges A.; Dunai Z. A.; Laner-Plamberger S.; Streif D.; Strunk D.; Rohde E.; Gimona M. An in vitro potency assay for monitoring the immunomodulatory potential of stromal cell-derived extracellular vesicles. Int. J. Mol. Sci. 2017, 18 (7), 1413. 10.3390/ijms18071413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Silvestre D.; Garavelli S.; Procaccini C.; Prattichizzo F.; Passignani G.; De Rosa V.; Mauri P.; Matarese G.; de Candia P. CD4+ T-Cell Activation Prompts Suppressive Function by Extracellular Vesicle-Associated MicroRNAs. Front. Cell Dev. Biol. 2021, 753884. 10.3389/fcell.2021.753884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P.; Zhang B.; Ocansey D. K. W.; Xu W.; Qian H. Extracellular vesicles: a bright star of nanomedicine. Biomaterials 2021, 269, 120467. 10.1016/j.biomaterials.2020.120467. [DOI] [PubMed] [Google Scholar]
- Caponnetto F.; Manini I.; Skrap M.; Palmai-Pallag T.; Di Loreto C.; Beltrami A. P.; Cesselli D.; Ferrari E. Size-dependent cellular uptake of exosomes. Nanomedicine: Nanotechnology, Biology and Medicine 2017, 13 (3), 1011–1020. 10.1016/j.nano.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Sharma S.; Rasool H. I.; Palanisamy V.; Mathisen C.; Schmidt M.; Wong D. T.; Gimzewski J. K. Structural-mechanical characterization of nanoparticle exosomes in human saliva, using correlative AFM, FESEM, and force spectroscopy. ACS Nano 2010, 4 (4), 1921–1926. 10.1021/nn901824n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perissinotto F.; Rondelli V.; Senigagliesi B.; Brocca P.; Almásy L.; Bottyán L.; Merkel D. G.; Amenitsch H.; Sartori B.; Pachler K.; et al. Structural insights into fusion mechanisms of small extracellular vesicles with model plasma membranes. Nanoscale 2021, 13 (10), 5224–5233. 10.1039/D0NR09075A. [DOI] [PubMed] [Google Scholar]
- Montis C.; Salvatore A.; Valle F.; Paolini L.; Carlà F.; Bergese P.; Berti D. Biogenic supported lipid bilayers as a tool to investigate nano-bio interfaces. J. Colloid Interface Sci. 2020, 570, 340–349. 10.1016/j.jcis.2020.03.014. [DOI] [PubMed] [Google Scholar]
- Cheng J.; Nonaka T.; Wong D. T. Salivary exosomes as nanocarriers for cancer biomarker delivery. Materials 2019, 12 (4), 654. 10.3390/ma12040654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S.; Castorph S.; Konovalov O.; Jahn R.; Holt M.; Salditt T. In vitro study of interaction of synaptic vesicles with lipid membranes. New J. Phys. 2010, 12 (10), 105004. 10.1088/1367-2630/12/10/105004. [DOI] [Google Scholar]
- Corrado C.; Barreca M. M.; Zichittella C.; Alessandro R.; Conigliaro A. Molecular Mediators of RNA Loading into Extracellular Vesicles. Cells 2021, 10 (12), 3355. 10.3390/cells10123355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal J.; Arras G.; Colombo M.; Jouve M.; Morath J. P.; Primdal-Bengtson B.; Dingli F.; Loew D.; Tkach M.; Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. U. S. A. 2016, 113 (8), E968–E977. 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallal S.; Azimi A.; Wei H.; Ho N.; Lee M. Y. T.; Sim H.-W.; Sy J.; Shivalingam B.; Buckland M. E.; Alexander-Kaufman K. L. A comprehensive proteomic SWATH-MS workflow for profiling blood extracellular vesicles: a new avenue for glioma tumour surveillance. Int. J. Mol. Sci. 2020, 21 (13), 4754. 10.3390/ijms21134754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmati M.; Bukva M.; Böröczky T.; Buzás K.; Gyukity-Sebestyén E. The role of the metabolite cargo of extracellular vesicles in tumor progression. Cancer Metastasis Rev. 2021, 1203–1221. 10.1007/s10555-021-10014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Wei F.; Schafer C.; Wong D. T. Detection of tumor cell-specific mRNA and protein in exosome-like microvesicles from blood and saliva. PloS one 2014, 9 (11), e110641. 10.1371/journal.pone.0110641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari E.; Ferrarotti I.; Di Silvestre D.; Grisoli P.; Barzon V.; Balderacchi A.; Torre M. L.; Rossi R.; Mauri P.; Corsico A. G.; et al. Adipose mesenchymal extracellular vesicles as alpha-1-antitrypsin physiological delivery systems for lung regeneration. Cells 2019, 8 (9), 965. 10.3390/cells8090965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan S.; Kang M.-H.; Jeyaraj M.; Qasim M.; Kim J.-H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 2019, 8 (4), 307. 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin E. Z.; Bratman S. V. Bioactive DNA from extracellular vesicles and particles. Cell Death Dis. 2020, 11 (7), 584. 10.1038/s41419-020-02803-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boilard E. Thematic Review Series: Exosomes and Microvesicles: Lipids as Key Components of their Biogenesis and Functions Extracellular vesicles and their content in bioactive lipid mediators: More than a sack of microRNA. J. Lipid Res. 2018, 59 (11), 2037–2046. 10.1194/jlr.R084640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R.; Greening D. W.; Chen M.; Rai A.; Ji H.; Takahashi N.; Simpson R. J. Surfaceome of exosomes secreted from the colorectal cancer cell line SW480: peripheral and integral membrane proteins analyzed by proteolysis and TX114. Proteomics 2019, 19 (8), 1700453. 10.1002/pmic.201700453. [DOI] [PubMed] [Google Scholar]
- Romancino D. P.; Buffa V.; Caruso S.; Ferrara I.; Raccosta S.; Notaro A.; Campos Y.; Noto R.; Martorana V.; Cupane A.; et al. Palmitoylation is a post-translational modification of Alix regulating the membrane organization of exosome-like small extracellular vesicles. Biochim. Biophys. Acta, Gen. Subj. 2018, 1862 (12), 2879–2887. 10.1016/j.bbagen.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Grava M.; Helmy S.; Gimona M.; Parisse P.; Casalis L.; Brocca P.; Rondelli V. Calorimetry of extracellular vesicles fusion to single phospholipid membrane. Biomol. Concepts 2022, 13 (1), 148–155. 10.1515/bmc-2022-0011. [DOI] [PubMed] [Google Scholar]