Abstract
Introduction:
There is mixed evidence as to whether nicotine vaping products (NVPs) can help adults who smoke transition away from cigarettes. This study investigated if rates of self-reported attempts to quit smoking and smoking cessation over a period of either 18 or 24 months differed between respondents who initiated nicotine vaping versus those who did not. Outcome comparisons were made between those who: (1) initiated vaping vs. those who did not; (2) initiated daily or non-daily vaping vs. those who did not; and (3) initiated daily or non-daily vaping between surveys and continued to vape at follow-up (daily or non-daily) vs. those who did not initiate vaping.
Methods:
This cohort study included 3516 respondents from the ITC Four Country Smoking and Vaping Surveys (Australia, Canada, England, US), recruited at Wave 1 (2016) or 2 (2018) and followed up at Wave 2 (12 months) and/or 3 (2020, 24 months). Adults who smoked daily at baseline and did not have a history of regular vaping were included. Initiation of vaping was defined as beginning to vape at least monthly between surveys. Respondents indicated whether they made an attempt to quit smoking between surveys. Smoking cessation was defined as those who self-reported no longer smoking cigarettes at follow-up.
Results:
Relative to those who did not initiate vaping, any daily vaping was associated with a greater likelihood of smokers making a cigarette quit attempt (p<0.001) and quitting smoking (p<0.001). Among smokers who attempted to quit smoking, any daily vaping was associated with a greater likelihood of being abstinent from smoking at follow-up (p=0.001). Respondents who initiated vaping between surveys and were vaping daily at follow up were significantly more likely to have attempted to quit smoking (p<0.001) and to have quit smoking (p<0.001) than those who did not initiate vaping. Respondents who initiated non-daily vaping did not differ significantly from those who did not initiate vaping on any of the outcome measures.
Conclusions:
Daily NVP use was associated with increased attempts to quit smoking and abstinence from smoking cigarettes. These findings are consistent with the concept that complete cigarette substitution may be more likely to be achieved when smokers vape nicotine daily.
Keywords: e-cigarettes, nicotine vaping products, tobacco, smoking cessation
1. Introduction
Over the last decade, the tobacco and nicotine product landscape has undergone dramatic changes with the emergence of non-combustible alternative nicotine delivery systems (Bozier et al., 2020; Hefler, 2018; National Academies of Sciences, Engineering and Medicine (NASEM), 2018). The most popular and rapidly growing class of these products are nicotine vaping products (NVPs, commonly known as e-cigarettes) (Foundation for a Smoke-Free World, 2018; Jones, 2019; NASEM, 2018). Evidence suggests that completely substituting NVPs for combustible cigarettes greatly reduces exposure to numerous toxicants and carcinogens (NASEM, 2018). However, since their emergence onto the global market, NVPs have been heavily debated, mainly as to whether or not they yield a net benefit to population health (Berridge, 2014; Hatsukami et al., 2020; Henningfield et al., 2018; US Department of Health & Human Services (HHS), 2020; Warner, 2019; WHO, 2021). With respect to people who smoke, the question of interest, is whether NVPs can help transition them away from smoking cigarettes, which are the deadliest form of nicotine delivery.
A recent Cochrane systematic review considered evidence from both randomized controlled trials (RCTs) and observational studies and concluded that there is “moderate-certainty evidence that e-cigarettes with nicotine increase quit rates compared to nicotine replacement therapy (NRT) and compared to e-cigarettes without nicotine” (Hartmann-Boyce et al., 2021). RCTs have been more consistent in finding improved smoking cessation rates for those who vape nicotine (Chan et al., 2021; Hajek et al., 2019; McRobbie et al., 2014; Myers Smith et al., 2021), while observational studies have produced mixed conclusions on the effectiveness of NVPs for smoking cessation (Beard et al., 2020; Benmarhnia et al., 2018; Brose et al., 2015; Coleman et al., 2018; Glasser et al., 2019; Hitchman et al., 2015; Johnson et al., 2019; Kulik et al., 2018; McDermott et al., 2021; Levy et al., 2017; Pierce et al., 2020). For example, two longitudinal studies of US adults in the Population Assessment of Tobacco and Health (PATH) Study, reported that there were no differences in rates of smoking cessation among those who used NVPs, NRT, or non-NRT medication (Kaplan et al., 2021; Pierce et al., 2020). One of the studies also found that NVPs were not associated with increased smoking cessation relative to those who did not use an aid (Pierce et al., 2020). The other study found that none of the exclusive cigarette smokers who used NVPs to quit smoking switched to exclusive NVP use, but 37.6% became dual users of NVPs and cigarettes (Kaplan et al., 2021). An important limitation of this latter study however is that it only tested whether smokers who used NVPs to quit differed from those who used approved pharmacotherapy such as NRT or prescription medication (varenicline or bupropion), and did not compare whether vaping increased quitting smoking relative to those who did not use an aid at all. In contrast, another PATH study that took vaping stability (frequency and consistency) into account, found that smokers who were not vaping across all survey waves, and those who were unstable in their use frequency (e.g., non-daily vaping across all waves or inconsistent use across time), were respectively 33% and 47% less likely to quit smoking compared to those who were vaping daily or who increased to daily use over three waves of data (Glasser et al., 2019). Other observational studies that have considered vaping frequency have also found higher smoking cessation rates for those who vape more frequently (daily) relative to those who did not vape (Coleman et al., 2019; Farsalinos et al., 2020; Hitchman et al, 2015; Kulik et al., 2018; Levy et al., 2018; McDermott et al., 2021; Wang et al., 2021), whereas less-than-daily use was associated with less quitting (Wang et al., 2021). Thus, taking into account this recent literature, vaping frequency appears to be a critical measure in gauging the nature of the relationship between NVP use and smoking cessation outcomes.
The question of whether NVPs can contribute to success in quitting smoking may be related to different mechanisms. For example, some smokers may be keen to use NVPs as a cessation aid (either in place of approved therapies, or in combination with other aids) and plan to move away from all nicotine use over time, whereas other smokers may decide to use a NVP as a complete and permanent replacement of cigarettes, with no intention to stop vaping nicotine. The latter ‘substitution’ mechanism may suggest that if NVPs are an adequate replacement (e.g., they are as satisfying and/or effective in their nicotine delivery relative to regular cigarettes), then smokers would be able to completely switch from smoking to vaping nicotine. This concept could also predict that those who smoke daily and take up daily nicotine vaping could increase their odds of quitting cigarettes.
At this time, more population-level evidence using rigorous and robust study designs are needed to help determine if vaping initiation and frequency are associated with changes in smoking behavior across time, most notably among those who are highly dependent on nicotine. This international cohort study investigated if rates of self-reported attempts to quit smoking and smoking cessation over a period of either 18 or 24 months differed between adults who smoked cigarettes daily at baseline and subsequently initiated vaping (NVP use) versus (vs.) those who did not. Specifically, outcome comparisons were made between those who: (1) initiated nicotine vaping vs. those who did not initiate vaping; (2) initiated daily or non-daily vaping vs. those who did not initiate vaping; and (3) initiated daily or non-daily vaping between surveys and continued to vape at follow-up (daily or non-daily) vs. those who did not initiate vaping. Overall, our study extends the work of other published observational studies that have examined NVPs for smoking cessation, in that we have attempted to examine multiple outcomes, including quit attempts and quit success among those who self-reported a quit attempt, as well as among those who did not report a quit attempt, while also considering key important covariates.
2. Methods
2.1. Design, setting, and participants
The ITC Four Country and Smoking and Vaping (ITC 4CV) Survey is a longitudinal cohort study that consists of four parallel online surveys conducted in Canada, the United Sates (US), England, and Australia. In addition to respondents retained from the ITC Four Country Survey (the predecessor of ITC 4CV prior to 2016) (ITC, 2011), adults (≥18 years) were recruited by commercial panel firms in each country at Wave 1 (W1: July-November 2016) as a person who: (1) had smoked at least 100 cigarettes in lifetime and were currently smoking at least monthly or less than monthly but occasionally (referred to herein as ‘smokers’); (2) or had smoked at least 100 cigarettes in their life-time and had quit smoking within the previous 2 years; or (3) were currently vaping at least weekly (referred to herein as ‘vapers’). Respondents eligible because of their smoking or cessation status might vape only monthly or occasionally (less than monthly). The sample in each country was designed to be as representative as possible of cigarette smokers and vapers (e.g., by age, sex, and region). All Wave 1 respondents were invited back to complete the Wave 2 survey (February-July 2018), and Wave 2 respondents were invited to complete Wave 3 (February-June 2020). The overall sample retention rate was 45.2% at Wave 2 and 42.2% at Wave 3. Among at least monthly smokers, 46.4% were retained at Wave 2 and 41.6% were retained at Wave 3, and among at least weekly vapers, 41.2% were retained at Wave 2 and 36.9% at Wave 3. At each wave, new respondents were recruited (using the same eligibility criteria as mentioned above) to compensate for those lost to follow-up and thus maintain the overall sample sizes for each country/user group combination. In order to compensate for those lost to follow-up, longitudinal weights were applied to the data for analyses to account for those lost to attrition.
Eligible respondents for the current study included those who: (1) completed at least two consecutive surveys; (2) were smoking daily at their baseline measure; and (3) had never vaped, or did not a history of any regular vaping (had never vaped on at least a monthly basis) prior to their baseline measure. For this study, 51.7% of baseline respondents were retained at Wave 2 and 48.3% were retained at Wave 3. The final analytic sampled included 4612 observations (Table 1) from 3516 unique individuals (Table 2).
Table 1.
Observational distribution by recruitment and recontact status
| Recontacted at Wave 2 (2018) |
Recontacted at Wave 3 (2020) |
Total number of pooled outcome observations |
|
|---|---|---|---|
| Recruited in Wave 1 (2016) | 2469 | 1096 | |
| Recruited in Wave 2 (2018) | — | 1047 | |
| Total | 2469 | 2143 | 4612 |
Table 2.
Respondents’ baseline characteristics
| Overall (N=3516) |
Canada (n=999) |
US (n=733) |
England (n=986) |
Australia (n=798) |
|
|---|---|---|---|---|---|
| Wave of Recruitment | |||||
| Recruited from the 4C Survey (<=2015) | 29.5 | 24.6 | 61.3 | 8.5 | 32.5 |
| Recruited in Wave 1 of the 4CV Survey (2016) | 46.2 | 52.3 | 12.3 | 69.0 | 41.6 |
| Recruited in Wave 2 of the 4CV Survey (2018) | 24.3 | 23.1 | 26.5 | 22.5 | 25.9 |
| Sex | |||||
| Female | 52.6 | 55.7 | 52.1 | 51.2 | 51.1 |
| Male | 47.4 | 44.3 | 47.9 | 48.8 | 48.9 |
| Age group | |||||
| 18-24 | 4.1 | 7.5 | 3.8 | 3.4 | 1.0 |
| 25-39 | 14.6 | 16.3 | 14.6 | 13.6 | 13.7 |
| 40-54 | 33.3 | 35.0 | 22.6 | 36.5 | 37.1 |
| 55+ | 48.0 | 41.1 | 58.9 | 46.5 | 48.2 |
| Income | |||||
| Low | 33.7 | 39.1 | 35.2 | 24.7 | 36.5 |
| Moderate | 32.6 | 24.5 | 35.1 | 47.1 | 22.6 |
| High | 27.2 | 28.1 | 28.9 | 19.0 | 34.6 |
| Not stated | 6.5 | 8.2 | 0.8 | 9.2 | 6.4 |
| Education | |||||
| Low | 37.7 | 33.7 | 40.1 | 39.9 | 37.6 |
| Moderate | 39.2 | 43.6 | 40.0 | 34.1 | 39.2 |
| High | 22.5 | 22.3 | 19.9 | 24.1 | 22.9 |
| Not stated | 0.7 | 0.3 | 0.0 | 1.9 | 0.3 |
| Cigarettes smoked/day (categorical) | |||||
| ≤ 10 cig/day | 35.2 | 37.8 | 42.8 | 34.8 | 25.3 |
| 11-20 cig/day | 47.9 | 44.5 | 41.7 | 51.6 | 53.3 |
| 21+ cig/day | 15.8 | 16.6 | 13.4 | 12.5 | 21.2 |
| Not stated | 1.1 | 1.0 | 2.0 | 1.1 | 0.3 |
| Cigarettes smoked/day (continuous) Mean (SD) | 15.3 (8.3) | 14.9 (8.3) | 14.8 (8.9) | 14.9 (7.5) | 17.0 (8.6) |
| Time to first cigarette | |||||
| > 60 min or not stated | 25.7 | 27.4 | 27.4 | 25.8 | 21.8 |
| 31-60 min | 13.0 | 13.0 | 12.6 | 13.1 | 13.3 |
| 6-30 min | 44.0 | 41.9 | 39.3 | 44.8 | 49.9 |
| < 5 min | 17.3 | 17.6 | 20.7 | 16.3 | 15.0 |
| Plans to quit smoking | |||||
| No/don’t know | 37.1 | 26.7 | 42.7 | 48.1 | 31.3 |
| Yes | 62.9 | 73.3 | 57.3 | 51.9 | 68.7 |
| Recent quit attempt | |||||
| No/don’t know | 62.5 | 55.5 | 68.3 | 71.5 | 54.8 |
| Yes | 37.5 | 44.5 | 31.7 | 28.5 | 45.2 |
| Vaping initiation by follow-up | |||||
| Initiated daily vaping | 10.9 | 10.3 | 8.2 | 15.8 | 8.0 |
| Initiated non-daily vaping | 10.3 | 11.0 | 9.3 | 14.0 | 5.8 |
| Did not initiate vaping | 78.8 | 78.7 | 82.5 | 70.2 | 86.2 |
Data are unweighted and unadjusted. All respondents included (eligible) for this study were limited to “exclusive daily smokers” who were not vaping at least monthly at the time of their first survey. US: United States; SD: Standard deviation. Plans to quit smoking: ‘yes’ (within the next month; between 1-6 months from now; sometime in the future, beyond 6 months) vs. ‘no’ (not planning to quit; don’t know). Recent quit attempt: Since you completed the last survey [18 months between Wave 1 to Wave 2 and 24 months between Wave 2 to Wave 3], have you tried to stop smoking?
The study was reviewed and cleared by research ethics committees in each country, and all participants provided informed consent. Further details about the ITC 4CV methods are described elsewhere (ITC Project, 2018; ITC Project, 2021; Thompson et al., 2019).
2.2. Measures
The surveys, with original response options, can be found at the ITC Project website: https://itcproject.org/surveys/. All measures are based on self-report at the time of the survey.
The following variables were used in the current study:
2.2.1. Smoking and vaping status
Survey questions about current smoking and vaping status can be found in Box 1.
Box 1.
-
All respondents were asked at baseline and follow-up:
How often, if at all, do you CURRENTLY smoke ordinary cigarettes (either factory-made/packet or roll-your-own)?- Daily
- Less than daily, but at least once a week
- Less than weekly, but at least once a month
- Less than monthly, but occasionally
- I have quit smoking
- I have never been a smoker
- All respondents were asked at baseline and follow-up:
-
Have you ever used an e-cigarette or vaping device, even one time?
- Yes
- No
- I have never heard of e-cigarettes/ vaping devices
If ‘yes’ to 2a, then respondents were asked: - How often, if at all, do you CURRENTLY use e-cigarettes/ vaping devices (i.e. vape)?
- Daily
- Less than daily, but at least once a week
- Less than weekly, but at least once a month
- Less than once a month, but occasionally
- Not at all
-
-
If a respondent did not answer ‘daily’ to 2b, they were subsequently asked:
At the time when you were vaping most often, how often did you vape?- Daily
- Less than daily, but at least once a week
- Less than weekly, but at least occasionally
- I have only tried vaping a few times, but more than once
- I have only ever tried vaping once
-
Respondents who were vaping daily at follow-up were asked to self-report the nicotine concentration:
What is the nicotine strength of the e-liquid you current use most?- None (0%) / None (0 mg/ml nicotine)
- Less than 1% / Less than 10 mg/ml
- 1 to 1.9% / 10-19 mg/ml
- 2 to 2.9% / 20-29 mg/ml
- 3 to 3.9% / 30-39 mg/ml
- 4 to 4.9% / 40-49 mg/ml
- 5% or more / 50 mg/ml or more
2.2.2. Covariates (assessed at baseline)
Sociodemographic variables:
Sociodemographic data were collected by the commercial panel firms and verified at the time of survey completion, including respondents’ age, sex, highest level of education, annual household income, and country of residence (see Table 1 for categorization of the variables).
Cigarette dependence:
Respondents were asked to report how many cigarettes on average they smoke per day (CPD). CPD was used as a continuous variable in all models. Additionally, respondents were asked about their ‘time to first cigarette’ (TTFC): How soon after waking do you usually smoke your first cigarette? This was categorized as: ‘< 5 minutes’; ‘6-30 minutes’; ‘31-60 minutes’; or ‘> 60 minutes’; ‘don’t know’; ‘not stated’. The latter two groups were marked as missing in the adjusted models.
Quit attempt prior to baseline measure:
Respondents were asked if they had made a quit attempt prior to their baseline survey (‘yes’ vs. ‘no/don’t know’). At Wave 1, respondents were asked if they had tried to stop smoking in the last 18 months, and at Wave 2 (baseline) if they had tried to quit in the last 24 months.
Plans to quit smoking:
Are you planning to quit smoking? This variable was coded as: ‘yes planning to quit’ vs. ‘not planning to quit/don’t know’.
Nicotine replacement therapy (NRT) use:
Are you currently using any nicotine replacement product(s), and if so, how often? Responses were categorized as: ‘daily use’,’ non-daily use’ or ‘no NRT use’.
Time-in-sample (TIS):
The analyses controlled for time-in-sample (TIS), the number of waves that the respondent had completed. TIS has been found to be related to differences in responses to a number of outcome variables in past ITC studies. Methodological details of TIS are presented elsewhere (Thompson et al., 2015).
2.2.3. Follow-up measures
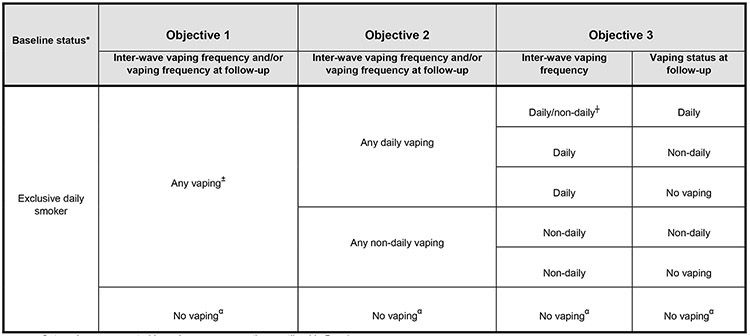
Initiation of vaping between baseline and follow-up (objective 1):
Initiation of vaping by respondents (‘initiated vaping’) was defined as those who reported that they began vaping at least monthly between baseline and follow-up. Respondents who initiated and stopped vaping between surveys were classified as ‘initiated vaping’. Respondents ‘did not initiate vaping’ if they did not have a history of regular vaping (had never vaped or previously vaped less than monthly) at baseline, and did not begin vaping at least monthly between baseline and follow-up. There was no minimum specified amount of time required for a respondent to qualify as currently vaping, as long as they indicated current use (at least monthly) at the time of completing their follow-up survey.
Vaping frequency between baseline and follow-up (objective 2):
This measure combined inter-wave vaping frequency and current vaping frequency at follow-up. Respondents were categorized into three groups: ‘initiated any daily vaping’ (current daily vaping at follow-up and/or vaped daily between surveys); ‘initiated any non-daily vaping’ (current weekly or monthly vaping at follow-up, and/or vaped either weekly or monthly between surveys); or ‘did not initiate vaping’ (vaped less than monthly or not at all between surveys).
Inter-wave vaping frequency and vaping frequency at follow-up (objective 3):
For this outcome, we further unfolded vaping frequency, and examined inter-wave vaping (daily, non-daily vs. no vaping) and vaping frequency at follow-up (daily, non-daily vs. no vaping). Respondents were categorized into six groups: (1) initiated daily or non-daily vaping between surveys and were currently vaping daily at follow-up (either increased vaping or remained a daily vaper); (2) initiated daily vaping between surveys but were currently vaping non-daily at follow-up (reduced vaping); (3) initiated non-daily vaping between surveys and were still vaping non-daily at follow-up (no change, remained a non-daily vaper); (4) initiated daily vaping between surveys but were not vaping at follow-up (stopped vaping); (5) initiated non-daily vaping but were not vaping at follow-up (stopped vaping); (6) did not initiate vaping at all.
Respondents who were vaping at least monthly at follow-up (regardless of their inter-wave vaping frequency) were asked if their NVP contained nicotine or not. Those who were vaping without nicotine (none: 0% or 0mg/ml) were classified as non- vapers at their follow-up measure (not using nicotine in their NVP) (n=59).
2.2.4. Outcome variables
Rates of making an attempt to quit smoking cigarettes (referred to herein now as “quit attempt”) and quit smoking cigarettes (referred to herein now as ‘smoking cessation’ or ‘quit smoking’) were assessed over a follow-up period of either 18 or 24 months (there were 18 months between 2016 and 2018 and 24 months between 2018 and 2020). The average length of time between baseline and follow-up was 18.8 months and for Waves 1/2 and 23.4 months for Waves 2/3.
Quit attempt between baseline and follow-up: This measure was self-reported using the following question: Since you completed the last survey [18 months between Wave 1 and Wave 2 and 24 months between Wave 2 and Wave 3], have you tried to stop smoking? This variable was coded as: ‘yes’ vs. ‘no/don’t know’.
Smoking cessation: Respondents were defined as having quit smoking if they reported that they were not smoking or cut down to smoking less than monthly at the time of their follow-up measure. Smoking cessation was a point-prevalence estimate, and did not take into account the length of time since quitting smoking cigarettes. We assessed cessation first among those who made a quit attempt (quit success) and then among the entire sample (regardless of a self-reported quit attempt or not).
2.3. Analyses
Unweighted descriptive statistics were used to describe baseline characteristics of the sample. All other analyses were conducted on weighted data. Generalized estimating equations (GEE) were used to generate regression models, and the predicted marginal standardization method (PREDMARG) was used to generate marginal estimates of the outcomes (Muller et al., 2014), while accounting for the within-person correlation over time. Analyses accounted for the complex survey design by incorporating strata and weights. Statistical significance and confidence intervals were computed at the 95% confidence level. All analyses were conducted in SAS-Callable SUDAAN (Version 11).
The first analysis (objective 1) examined whether there were differences between smokers who initiated vaping vs. those who did not initiate vaping on: (1) having made a quit attempt; and (2) quitting smoking (first among those who made a quit attempt and then among all respondents) (Model 1). Second (objective 2), we analyzed whether these outcomes differed between the three groups (combined measures of vaping between surveys and at follow-up) (Model 2). The latter results were displayed overall and then by country to determine if the pattern of vaping frequency was similar to the overall estimates across the four countries combined. Third (objective 3), using the six groups defined above, we tested if those who did not initiate vaping differed from the other five groups (combinations of frequency of inter-wave and current vaping at follow-up) in making a quit attempt or quitting smoking (Model 3). The findings were only analyzed among all respondents, and not by country, due to small sample sizes in some of the groups.
Covariates in all models included: age group, sex, country of residence, education, income, cigarette dependence (TTFC and CPD), plans to quit smoking, made a quit attempt prior to baseline, TIS, wave of recruitment, and NRT use at follow-up.
3. Results
3.1. Respondents’ characteristics
The (unweighted) baseline sample characteristics of smokers participating in this study are presented in Table 2. Among all respondents (N=3516), 21.2% initiated vaping (at least monthly), and 78.8% did not initiate vaping between surveys. England had the highest percentage of adult smokers who initiated vaping (29.8%) and Australia had the lowest (13.8%). Among exclusive smokers who did not initiate vaping between baseline and follow-up, 11.6% had prior to baseline vaped more than once or occasionally (less than monthly) and 3.4% had tried vaping only once.
3.2. Quit smoking attempts and smoking abstinence among all respondents
Among all 3516 baseline exclusive smokers, 36.9% reported making at least one attempt to quit smoking during the follow-up period. Of those who made a quit attempt, 28.3% self-reported having quit smoking at their follow-up measure. Overall, regardless of a self-reported quit attempt or not, 10.5% of all respondents reported that they had quit smoking by follow-up.
3.3. Quit smoking attempts and stopping smoking among baseline exclusive daily smokers based on vaping status and frequency
Table 3 shows the results for Model 1 (objective 1) and Model 2 (objective 2).
Table 3.
The examination of quit smoking attempts and quitting smoking as a function of vaping status and frequency
| Vaping status between baseline and follow-up | Quit attempt | Quit smoking among, smokers who made a quit attempt (n=1690) |
Quit smoking (overall) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Overall (N=4612) | 1690 | 36.9% | 485 | 28.3% | 485 | 10.5% |
| Model 1 | ||||||
| Did not initiate vaping* (n=3745) | 1267 | 36.3% | 351 | 27.3% | 351 | 9.8% |
| Initiated vaping after baseline (≥ monthly) (n=867) | 406 | 45.0% | 126 | 32.3% | 126 | 15.7% |
| p-value | <0.001 | 0.16 | <0.001 | |||
| aOR (95% CI) for any vaping vs. no vaping | 1.60 (1.25 - 2.06) | 1.30 (0.91- 1.86) | 1.76 (1.27- 2.44) | |||
| Model 2 | ||||||
| Did not initiate vaping* (n=3745) | 1267 | 36.3% | 351 | 27.1% | 351 | 9.8% |
| Initiated any daily vaping† (n=456) | 249 | 53.8% | 106 | 40.6% | 106 | 23.4% |
| p-value | <0.001 | 0.001 | <0.001 | |||
| aOR (95% CI) for daily vaping vs. no vaping | 2.52 (1.83 - 3.47) | 1.95 (1.29 - 2.94) | 3.00 (2.08 - 4.33) | |||
| Initiated any non-daily vaping† (n=411) | 157 | 34.9% | 20 | 16.7% | 20 | 6.3% |
| p-value | 0.69 | 0.053 | 0.11 | |||
| aOR (95% CI) for non-daily vaping vs. no vaping | 0.93 (0.64 −1.34) | 0.51 (0.26 - 1.01) | 0.61 (0.33 - 1.11) | |||
Estimates for each outcome are weighted and adjusted for age, sex, country of residence, education, income, cigarette dependence, plans to quit smoking, quit attempt prior to baseline, TIS, wave of recruitment, and NRT use at follow-up. Vaping status between baseline and follow-up: the outcome takes into account the frequency of vaping between surveys and at follow-up.
Sample includes anyone who vaped daily/non-daily between surveys, as well as at their follow-up measure.
Reference group: includes those who vape less than monthly or not at all; ‘n’ are the number of observations in the models for each outcome and are not the number of unique respondents in the sample. Bolded statistics denote statistical significance.
3.3.1. Quit smoking attempts
Respondents who initiated vaping between surveys were more likely to have made a quit attempt (45.0%; aOR=1.60, 95% CI:1.25-2.06) compared to those who did not initiate vaping (36.3%).
Based on frequency of vaping, those who initiated daily vaping were significantly more likely to have made a quit attempt (53.8%; aOR=2.52, 95% CI:1.83-3.47) compared to those who did not initiate vaping (36.3%). Those who initiated non-daily vaping did not differ in making an attempt (34.9%; aOR=0.93, 95% CI:0.64 −1.34) than those who did not initiate vaping.
3.3.2. Smoking cessation (quit success) among those who tried to quit smoking
Among respondents who self-reported that they made a quit attempt, there were no significant differences between those who initiated vaping (32.3%) and those who did not (27.3%) (aOR=1.30; 95% CI:0.91-1.86) in quitting smoking.
Based on frequency of vaping, those who initiated daily vaping and made a quit attempt, were significantly more likely to have quit smoking (40.6%; aOR=1.95, 95% CI:1.29-2.94) compared to those who made a quit attempt but did not initiate vaping (27.1%). Of those who were vaping non-daily and made a quit attempt, 16.7% quit smoking by follow-up, which trended towards a lower likelihood of having quit smoking relative to those who were not vaping (aOR=0.51, 95% CI: 0.26-1.01).
3.3.3. Smoking cessation among all respondents
Regardless of whether respondents made a quit attempt or not, those who initiated vaping were more likely to have quit smoking by follow-up (15.7%; aOR=1.76; 95% CI: 1.27- 2.44) compared to those who did not initiate vaping (9.8%).
Respondents who initiated daily vaping were significantly more likely to have quit smoking (23.4%; aOR=3.00, 95% CI: 2.08-4.33) than those who did not initiate vaping, but non-daily vapers (6.3%; aOR=0.61, 95% CI: 0.33-1.11) did not differ significantly from those who did not initiate vaping (9.8%).
Figures 2a-c show quit smoking attempts and smoking abstinence among baseline exclusive daily smokers by country and vaping status. Similar to the overall findings, the same pattern within each country was found, where those who initiated daily vaping after their baseline measure had higher rates of making an attempt to quit smoking and quitting smoking relative to those who initiated non-daily vaping or did not initiate vaping.
Figure 2a.
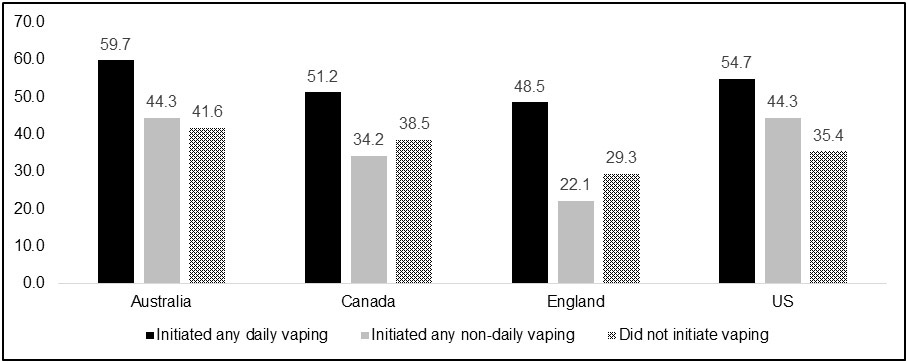
Proportion of daily cigarette smokers who attempted to quit smoking, by country and vaping status
3.4. Quit smoking attempts and smoking abstinence among baseline exclusive daily smokers based on inter-wave vaping frequency and vaping frequency at follow-up
Table 4 shows the results for quit attempts and smoking cessation based on vaping frequency between waves in combination with vaping frequency at follow-up (six groups, objective 3).
Table 4.
The examination of quit attempts and quitting smoking based on patterns of vaping frequency from initiation to follow-up (Model 3)
| Vaping frequency between surveys |
Vaping frequency at follow-up |
n | % | aOR (95% CI) |
|---|---|---|---|---|
| Made a quit attempt | ||||
| Daily/non-daily* | Daily (n=185) | 126 | 63.3 | 4.19 (2.58-6.82) |
| Daily | Non-daily (n=42) | 22 | 55.6 | 2.78 (1.06-7.27) |
| Non-daily | Non-daily (n=136) | 49 | 32.5 | 0.81 (0.43-1.52) |
| Daily | Not vaping (n=205) | 101 | 44.8 | 1.58 (1.02-2.46) |
| Non-daily | Not vaping (n=260) | 108 | 36.1 | 0.99 (0.64-1.53) |
| Not vaping | Not vaping (n=3701) | 1267 | 36.3 | Reference |
| Quit smoking (among those who made a quit attempt) | ||||
| Daily/non-daily* | Daily (n=140) | 82 | 58.2 | 4.42 (2.60-7.52) |
| Daily | Non-daily (n=26) | 1 | 12.3 | 0.36 (0.04-2.97) |
| Non-daily | Non-daily (n=56) | 4 | 11.5 | 0.33 (0.09-1.26) |
| Daily | Not vaping (n=101) | 23 | 25.1 | 0.91 (0.45-1.86) |
| Non-daily | Not vaping (n=108) | 16 | 19.4 | 0.63 (0.29-1.38) |
| Not vaping | Not vaping (n=1267) | 351 | 26.7 | Reference |
| Quit smoking (all respondents) | ||||
| Daily/non-daily* | Daily (n=199) | 82 | 39.0 | 6.77 (4.27-10.75) |
| Daily | Non-daily (n=46) | 1 | 9.2 | 0.93 (0.13-6.76) |
| Non-daily | Non-daily (n=143) | 4 | 4.4 | 0.41 (0.12-1.38) |
| Daily | Not vaping (n=205) | 23 | 12.2 | 1.30 (0.72-2.33) |
| Non-daily | Not vaping (n=261) | 16 | 7.5 | 0.73 (0.38-1.42) |
| Not vaping | Not vaping (n=3709) | 351 | 9.8 | Reference |
Estimates are weighted and adjusted for age, sex, country of residence, education, income, cigarette dependence, plans to quit smoking, quit attempt prior to baseline, TIS, wave of recruitment, and NRT use at follow-up. Respondents who were vaping daily at their follow-up measure were not asked about their vaping frequency between surveys. ‘n’ are the number of observations in the models for each outcome and are not the number of unique respondents in the sample. Bolded statistics denote statistical significance. aOR: Adjusted odds ratio; CI: Confidence interval.
Inter-wave vaping status (vaping between surveys) / vaping status at follow-up: Respondents were categorized into six groups based on the above vaping frequency and timing measures. Respondents vaping frequency were considered after initiation (between surveys) and at their follow-up measure (current vaping frequency). They were as follows: (1) initiated daily or non-daily vaping between surveys and were currently vaping daily at follow-up (either increased vaping or remained a daily vaper); (2) initiated daily vaping between surveys but were currently vaping non-daily at follow-up (reduced vaping); (3) initiated non-daily vaping between surveys and were still vaping non-daily at follow-up (no change, remained a non-daily vaper); (4) initiated daily vaping between surveys but were not vaping at follow-up (stopped vaping); (5) initiated non-daily vaping but were not vaping at follow-up (stopped vaping); (6) did not initiate vaping at all.
3.4.1. Quit smoking attempts
Respondents who: (1) initiated vaping (daily/non-daily) and were currently vaping daily at follow-up (63.3%; aOR=4.19, 95% CI: 2.58-6.82); (2) vaped daily between surveys and reduced to non-daily vaping by follow-up (55.6%; aOR=2.78, 95% CI:1.06-7.27); and (3) vaped daily between surveys and were not currently vaping at follow-up (44.8%; aOR=1.58, 95% CI:1.02-2.46) were significantly more likely to have made a quit attempt compared to those who did not initiate vaping (36.3%). There were no significant differences in the likelihood of making a quit attempt between respondents who vaped non-daily between surveys and at follow-up (32.5%; aOR=0.81, 95% CI: 0.43-1.52) and those who vaped non-daily between surveys and were not currently vaping at follow-up (36.1%; aOR=0.99, 95% CI: 0.64-1.53) relative to those who did not initiate vaping (36.3%).
3.4.2. Smoking cessation (quit success) among those who tried to quit smoking
Among respondents who made a quit attempt, only those who initiated vaping, and were currently vaping daily at follow-up were significantly more likely to have quit smoking by follow-up (58.2%; aOR=4.42, 95% CI:2.60-7.52) compared to those who did not initiate vaping (26.7%). The other groups who initiated vaping at any frequency, but were not vaping daily at follow-up had lower rates of having quit smoking after a quit attempt (ranging from 11.5% to 25.1%) compared to those who did not initiate vaping, but these comparisons were not statistically significant.
3.4.3. Smoking cessation among all respondents
Regardless of whether respondents made a quit attempt or not, only those who initiated vaping and were currently vaping daily at follow-up were significantly more likely to have quit smoking (39.0%; aOR=6.77, 95% CI:4.27-10.75) compared to those who did not initiate vaping (9.8%). Rates of quitting smoking among the other groups who initiated vaping at any frequency, but were not vaping daily at follow-up ranged from 4.4% to 12.2%. They did not differ significantly from those who did not initiate vaping.
4. Discussion
This longitudinal study of adults who exclusively smoke daily in four countries, investigated whether the initiation and frequency of NVP use was associated with a greater likelihood of making an attempt to quit cigarette smoking and quitting smoking relative to those who did not initiate vaping. Our findings support a growing body of literature that daily nicotine vaping was associated with a greater likelihood of smokers making a quit attempt (Brose et al., 2015) and abstaining from smoking (Coleman et al., 2018; Glasser et al., 2019; Hitchman et al., 2015; Levy et al., 2017; McDermott et al., 2021; Wang et al., 2021), whereas less frequent vaping was not (Glasser et al., 2019; Wang et al., 2021). When the data were stratified by each of the four countries, the same pattern was found, such that daily vapers had higher rates of quit attempts and smoking cessation relative to less frequent vapers and non-vapers. We also found that smokers who initiated vaping and were currently vaping daily at follow-up were nearly seven times more likely to have quit smoking compared to those who did not initiate vaping. Additionally, our findings showed that smokers who initiated daily vaping between surveys, but then had reduced to non-daily use by follow-up, had a greater likelihood of making a quit attempt, but were not more likely to have quit smoking compared to non-vapers. These results appear to suggest that continued regular daily vaping is key predictor of successful smoking cessation.
While some smokers use NVPs to try and quit smoking, other motivations for use are involved as well, including saving money, perceived health benefits, reducing social stigma associated with smoking, and convenience, since NVPs may be possible to use in places where smoking is prohibited or frowned upon (e.g., inside the home, in the car, at work, and in other public places) (Glasser et al., 2017; Newcombe et al., 2021; Patel et al., 2019; Simonavicius et al., 2017; Vandrevala et al., 2017; Yong et al., 2019). Thus, the findings in this study may reflect inherent differences in the characteristics and motivations between those who vape daily versus occasionally. Studies that combine outcomes among vapers who are vaping at different frequencies, and may or may not be using NVPs to quit smoking, may be less capable of detecting possible effects of NVP use on quit attempts and abstinence rates.
Although there is no one magic treatment that will get all smokers to quit, it does appear that many smokers can benefit from vaping nicotine. This in part, is likely owing to the more rapid onset of nicotine delivery from newer generation devices that can be more helpful in reducing nicotine cravings than older and less efficient devices (Hajek et al., 2020; O'Connell et al., 2019; Wagener et al., 2017; Yingst et al., 2019). It is notable however, that that the majority of daily smokers in our study did not initiate NVP use, or initiated use but stopped using them. There could be several reasons for this, including that they do not want to give up cigarette smoking (McKeganey et al., 2017), worry about the risks of vaping and/or about the unknown risks of long-term use (Marques et al., 2021), misperceptions about the relative harmfulness of NVPs compared to cigarettes (Elton-Marshall et al., 2020; National Cancer Institute, 2019; Wilson et al., 2019; Yong et al., 2021), they have not been advised to do so by a healthcare provider (Gravely et al., 2019; Singh et al., 2017; Van Gucht et al., 2016; University of North Carolina Health Care System, 2016), they believe that NVPs are unreliable and/or too complex (McKeganey et al., 2017), dissatisfaction with vaping or NVPs were not found to be an effective substitute for cigarettes (Yong et al., 2019), restrictive regulatory policies (e.g., they are not legal to be sold and therefore there are accessibility barriers) (Lum et al., 2021; Morphett et al., 2019; Yong et al., 2017), and/or warnings from public health organizations not to vape as they have not been found to be safe and effective in helping smokers quit (American Lung Association, 2020; Gee et al., 2021; Joint Position Statement from Six Lung Societies, 2014; NASEM, 2018). In our survey, among respondents who did not initiate vaping between baseline and follow-up, about 15% of this sample had tried vaping or vaped occasionally (less than monthly) prior to their baseline survey. Reasons why these smokers who tried vaping nicotine did not continue with vaping, and whether they had ever tried or used approved pharmacotherapy (NRT and/or other prescription medications), should be further explored.
Another important area of investigation is to assess whether those who have quit smoking using a NVP plan to continue vaping permanently, or whether they intend to quit all nicotine use once they are abstinent from cigarettes. A study by Palmer et al. (2021) examined the level of interest in NVP discontinuation among US adults with and without a history of smoking and found that a majority of vapers (61%) expressed interest in eventually quitting vaping. Plans to quit vaping did however differ by history of smoking, where ex-smokers had the highest levels of intentions to quit vaping (66%) relative to dual users (59%) and never-smokers (55%). This should be further investigated. Notably, if ex-smokers do wish to quit or reduce their vaping at some point along their smoking cessation trajectory, information, resources, support, and treatment options should be provided by healthcare professionals.
4.1. Limitations
While this study has several strengths, including a large cohort sample of representative smokers from four countries, there are limitations to consider. First, the study design does not allow us to make a causal inference if NVPs were a successful quit aid since we do not know whether vaping was initiated before, during or after a quit attempt. For example, it is possible that some might have taken up vaping after they quit smoking as a way to prevent relapse. Second, respondents who were vaping daily at follow-up were not asked about their inter-wave vaping frequency (between surveys). Consequently, it is unclear if those who were vaping daily at follow-up began with daily use, or instead vaped less frequently and increased use across time. Third, we did not assess if smokers used other forms of cessation treatment between surveys. Fourth, the retrospective measurements in this study relied on respondent recall over a period of either 18 or 24 months, which may have impacted reports of having made a formal quit attempt and/or whether they tried to use a NVP during a quit attempt. It has been previously shown that some smokers have poor recall of quit attempts (particularly those of short duration) and cessation methods used during an attempt to quit smoking (Berg et al., 2010; Borland et al., 2012a,b; Chaiton et al. 2016; Hammond et al. 2004). As such, better validations of self-reported attempts to quit smoking are important to accurately identify population estimates of cessation and to increase the understanding of smoking trajectories.
5. Conclusion
This study found that among adults who smoke daily, and are likely highly dependent on nicotine, the initiation of daily NVP use was associated with increased quit attempts and abstinence from smoking. Those who continued to vape daily across time, were the most successful at quitting smoking. These findings are consistent with the concept that complete cigarette substitution may be more likely to be achieved when NVPs are used daily. The differing patterns of NVP use may reflect inherent differences in the characteristics and motivations of daily versus non-daily NVP use and/or differences in the types of vaping products used in observational studies. Therefore, more randomized trials are urgently needed to isolate the role of NVP product features, dosage, and duration of NVP use in changing smoking behavior, and to assess the true potential of NVPs as cigarette substitutes.
Figure 1. Respondents’ vaping status and frequency categorization for each of the study objectives.
Categories were created based on survey questions outlined in Box 1.
*Those who reported vaping at least monthly or had a history of more frequent vaping prior to their baseline measure were excluded from the analytical sample. ± ‘Any vaping’ were those who initiated vaping at least monthly. ┼ Inter-wave vaping frequency is unknown. α Reference group.
Figure 2b.
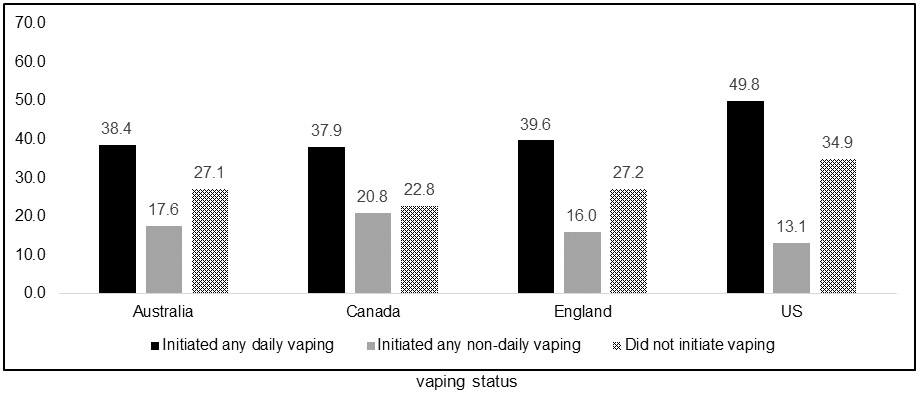
Proportion of daily cigarette smokers who quit smoking among those who made a quit attempt, by country and vaping status
Figure 2c.
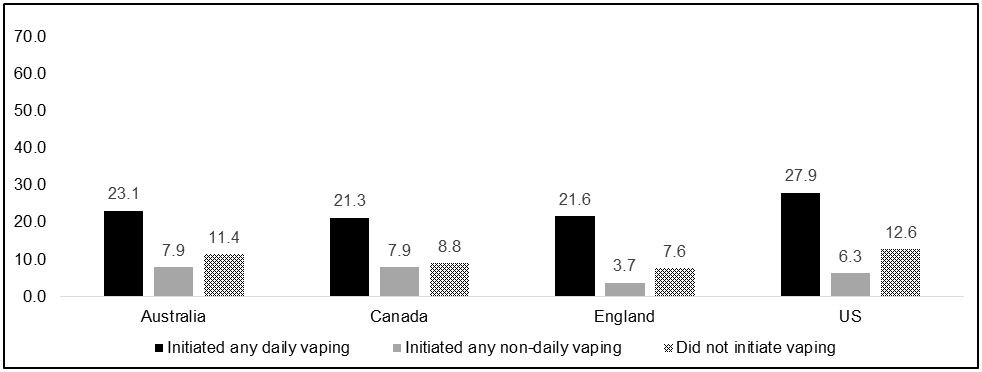
Proportion of daily cigarette smokers who quit smoking among all respondents, by country and vaping status
Highlights.
There is mixed evidence whether nicotine vaping products (NVPs) can help adults quit smoking.
Some evidence suggests that more frequent vaping is associated with increased abstinence from smoking.
We found that compared to daily smokers who did not initiate vaping, daily vaping was associated with a greater likelihood of quitting smoking.
Non-daily vaping was not associated with quit attempts or quit success compared to those who did not initiate vaping.
These findings demonstrate that complete cigarette substitution may be more likely achieved when daily smokers use NVPs daily.
Acknowledgments:
The authors would like to acknowledge and thank all those that contributed to the International Tobacco Control Four Country Smoking and Vaping Survey (ITC 4CV) Survey: all study investigators and collaborators, and the project staff at their respective institutions.
Funding:
This study was supported by grants from the National Cancer Institute of the US (P01CA200512), the Canadian Institutes of Health Research (FDN-148477), and by the National Health and Medical Research Council of Australia (APP1106451). Additional support to GTF was provided by a Senior Investigator Award from the Ontario Institute for Cancer Research and the Canadian Cancer Society O. Harold Warwick Prize. Additional support to AH and RJO was provided by a Tobacco Centers of Regulatory Science US National Cancer Institute grant (U54CA238110). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit for publication.
Footnotes
Ethics approval: The survey protocols and all materials, including the survey questionnaires, were approved by the Research Ethics committee at the University of Waterloo, Canada (ORE#20803/30570, ORE#21609/30878), King’s College London, UK (RESCM-17/18-2240), Cancer Council Victoria, Australia (HREC1603), University of Queensland, Australia (20160000330/HREC1603), Deakin University, Australia (DUHREC2018-346) and Medical University of South Carolina, US (waived due to minimal risk).
References
- Benmarhnia T, Pierce JP, Leas E, White MM, Strong DR, Noble ML, Trinidad DR (2018). Can e-cigarettes and pharmaceutical AIDS increase smoking cessation and reduce cigarette consumption? Findings from a nationally representative cohort of American smokers. Am J Epidemiol 187:2397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard E, West R, Michie S, Brown J (2020). Association of prevalence of electronic cigarette use with smoking cessation and cigarette consumption in England: a time-series analysis between 2006 and 2017 Addiction 115(5), 961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg CJ, An LC, Kirch M, Guo H, Thomas JL, Patten CA Ahluwalia JS, West R (2014). Failure to report attempts to quit smoking. Addictive Behaviors 35, 900–904. [DOI] [PubMed] [Google Scholar]
- Berridge V (2014). Electronic cigarettes and history. Lancet, 383(9936), 2204–2205. [DOI] [PubMed] [Google Scholar]
- Bozier J, Chivers EK, Chapman DG, Larcombe AN, Bastian NA, Masso-Silva JA, Byun MK, McDonald CF, Crotty Alexander LE, Ween MP (2020). The Evolving Landscape of e-Cigarettes: A Systematic Review of Recent Evidence. Chest, 157(5), 1362–1390. [DOI] [PubMed] [Google Scholar]
- Borland R, Partos TR, Yong HH, Cummings KM, Hyland A (2012a). How much unsuccessful quitting activity is going on among adult smokers? Data from the International Tobacco Control Four Country cohort survey. Addiction 107(3), 673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland R, Partos TR, Cummings KM (2012b). Systematic biases in cross-sectional community studies may underestimate the effectiveness of stop-smoking medications. Nicotine Tob Res 14(12), 1483–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley S, Green C (eds.) (2020). The Economics of Education: A Comprehensive Overview (2nd ed.). Difference-In-Differences. Academic Press. https://www.sciencedirect.com/topics/economics-econometrics-and-finance/difference-in-differences [Google Scholar]
- Brose LS, Hitchman SC, Brown J, West R, McNeill A (2015). Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction 110(7), 1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiton M, Diemert L, Cohen JE, Bondy SJ, Selby P, Philipneri A, Schwartz R (2016. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open 6(6):e011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan GCK, Stjepanović D, Lim C, Sun T, Shanmuga Anandan A, Connor JP, Gartner C, Hall WD, Leung J (2021). A systematic review of randomized controlled trials and network meta-analysis of e-cigarettes for smoking cessation. Addict Behav 119, 106912. [DOI] [PubMed] [Google Scholar]
- Coleman B, Rostron B, Johnson SE, Persoskie A, Pearson J, Stanton C, Choi K, Anic G, Goniewicz ML, Cummings KM, Kasza KA, Silveira ML, Delnevo C, Niaura R, Abrams DB, Kimmel HL, Borek N, Compton WM, Hyland A (2019). Transitions in electronic cigarette use among adults in the Population Assessment of Tobacco and Health (PATH) Study, Waves 1 and 2 (2013-2015). Tob control, 28(1), 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton-Marshall T, Driezen P, Fong GT, Cummings KM, Persoskie A, Wackowski O, Choi K, Kaufman A, Strong D, Gravely S, Taylor K, Kwan J, Bansal-Travers M, Travers M, Hyland A (2020). Adult perceptions of the relative harm of tobacco products and subsequent tobacco product use: Longitudinal findings from waves 1 and 2 of the population assessment of tobacco and health (PATH) study. Addictive behaviors, 106, 106337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Niaura R (2020). E-cigarettes and Smoking Cessation in the United States According to Frequency of E-cigarette Use and Quitting Duration: Analysis of the 2016 and 2017 National Health Interview Surveys. . Nicotine Tob Res 22(5), 655–662. [DOI] [PubMed] [Google Scholar]
- Gee RE, Boles WR, Smith DG (2021). E-Cigarettes: A Public Health Threat, Not a Population Health Intervention. Am J Public Health 111(2), 224–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser AM, Collins L, Pearson JL, Abudayyeh H, Niaura RS, Abrams DB, Villanti AC (2017). Overview of Electronic Nicotine Delivery Systems: A Systematic Review. Am J Prev Med 52(2), e33–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser AM, Vojjala M, Cantrell J, Levy DT, Giovenco DP, Abrams D, Niaura R (2021). Patterns of E-cigarette Use and Subsequent Cigarette Smoking Cessation Over 2 Years (2013/2014-2015/2016) in the Population Assessment of Tobacco and Health Study. Nicotine Tob Res 23(4), 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global trends in nicotine. Foundation for a Smoke-Free World. (2020). Retrieved September 23, 2021, from https://www.smokefreeworld.org/advancing-industry-transformation/global-trends-nicotine/
- Gravely S, Thrasher JF, Cummings KM, Ouimet J, McNeill A, Meng G, Lindblom EN, Loewen R, O'Connor RJ, Thompson ME, Hitchman SC, Hammond D, Heckman BW, Borland R, Yong HH, Elton-Marshall T, Bansal-Travers M, Gartner C, Fong GT (2019). Discussions between health professionals and smokers about nicotine vaping products: results from the 2016 ITC Four Country Smoking and Vaping Survey. Addiction 114 Suppl 1(Suppl 1), 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, Phillips-Waller A, Przulj D, Pesola F, Smith KM, Bisal N, Li J, Parrott S, Sasieni P, Dawkins L, Ross L, Goniewicz M, Wu Q, McRobbie HJ (2019). E-cigarettes compared with nicotine replacement therapy within the UK stop Smoking services: The TEC RCT. Health Technol Assess 23(43), 1–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, Pittaccio K, Pesola F, Myers Smith K, Phillips-Waller A, Przulj D (2020). Nicotine delivery and users' reactions to Juul compared with cigarettes and other e-cigarette products. Addiction 115(6), 1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D, McDonald PW, Fong GT, Borland R (2004). Do smokers know how to quit? Knowledge and perceived effectiveness of cessation assistance as predictors of cessation behaviour. Addiction 99(8), 1042–1048. [DOI] [PubMed] [Google Scholar]
- Hartmann-Boyce J, McRobbie H, Butler AR, Lindson N, Bullen C, Begh R, Theodoulou A, Notley C, Rigotti NA, Turner T, Fanshawe TR, Hajek P (2021). Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev 9(9), CD010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Carroll DM (2020). Tobacco harm reduction: Past history, current controversies and a proposed approach for the future. Prev Med 140, 106099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefler M (2018). The changing nicotine products landscape: time to outlaw sales of combustible tobacco products?. Tob Control 27(1), 1–2. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Higgins ST, Villanti AC (2018). Are we guilty of errors of omission on the potential role of electronic nicotine delivery systems as less harmful substitutes for combusted tobacco use?. Prev Med 117, 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute (NCI). (2020). Hints Briefs Number 42: Health Information National Trends Survey. Retrieved September 23, 2021, from https://hints.cancer.gov/docs/Briefs/HINTS_Brief_42.pdf
- Hitchman SC, Brose LS, Brown J, Robson D, McNeill A (2015). Associations Between E-Cigarette Type, Frequency of Use, and Quitting Smoking: Findings From a Longitudinal Online Panel Survey in Great Britain. Nic Tob Res 17(10), 1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITC Project. (2011, September). International Tobacco Control Policy Evaluation Survey (ITC): Four Country Project Waves 2-8 Technical Report, from https://itcproject.s3.amazonaws.com/uploads/documents/4c-w28-tech-report-sept.pdf
- ITC Project. (2020, January). ITC Four Country Smoking and Vaping Survey, Wave 2 (2018) Technical Report. University of Waterloo, Waterloo, Ontario, Canada; Medical University of South Carolina, Charleston, South Carolina, United States; Cancer Council Victoria, Melbourne, Australia; the University of Queensland, Australia; King’s College London, London, United Kingdom. Retrieved September 23, 2021, from https://itcproject.s3.amazonaws.com/uploads/documents/4CV2_Technical_Report_15Jan202.pdf
- ITC Project. (2021, July). ITC Four Country Smoking and Vaping Survey, Wave 3 (4CV3, 2020) Technical Report. University of Waterloo, Waterloo, Ontario, Canada; Medical University of South Carolina, Charleston, South Carolina, United States; Cancer Council Victoria, Melbourne, Australia; the University of Queensland, Australia; King’s College London, London, United Kingdom. Retrieved September 23, 2021, from https://itcproject.s3.amazonaws.com/uploads/documents/4CV3_Technical_Report_23Jul2021_2.pdf
- Johnson L, Ma Y, Fisher SL, Ramsey AT, Chen LS, Hartz SM, Culverhouse RC, Grucza RA, Saccone NL, Baker TB, Bierut LJ (2019). E-cigarette Usage Is Associated With Increased Past-12-Month Quit Attempts and Successful Smoking Cessation in Two US Population-Based Surveys. Nicotine Tob Res, 21(10), 1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint Position Statement from Six Lung Societies. (2014). E-Cigarettes, Oncology Times, 36 (15), 49–50. [Google Scholar]
- Jones L (2019). Vaping: How popular are e-cigarettes? BBC News. Retrieved September 23, 2021, from https://www.bbc.com/news/business-44295336 [Google Scholar]
- Kaplan B, Galiatsatos P, Breland A, Eissenberg T, Cohen JE (2021). Effectiveness of ENDS, NRT and medication for smoking cessation among cigarette-only users: a longitudinal analysis of PATH Study wave 3 (2015-2016) and 4 (2016-2017), adult data. Tob Control tobaccocontrol-2020–056448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza KA, Edwards KC, Tang Z, Stanton CA, Sharma E, Halenar MJ, Taylor KA, Donaldson EA, Hull LC, Bansal-Travers M, Limpert J, Zandberg I, Gardner LD, Hammad HT, Borek N, Kimmel HL, Compton WM, Hyland A (2020). Correlates of tobacco product cessation among youth and adults in the USA: findings from the PATH Study Waves 1-3 (2013-2016). Tob Control 29 (Suppl 3), s203–s215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik MC, Lisha NE, Glantz SA (2018). E-cigarettes Associated With Depressed Smoking Cessation: A Cross-sectional Study of 28 European Union Countries. Am J Prev Med 54(4), 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DT, Yuan Z, Luo Y, Abrams DB (2018). The Relationship of E-Cigarette Use to Cigarette Quit Attempts and Cessation: Insights from a Large, Nationally Representative U.S. Survey. Nic Tob Res 20(8), 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum A, Skelton E, Robinson M, Guillaumier A, Wynne O, Gartner C, Borland R, Baker A, Dunlop A, Wilkinson RB, Bonevski B (2021). Barriers and facilitators to using vaporised nicotine products as smoking cessation aids among people receiving treatment for substance use disorder. ddict Behav 124, 107097. [DOI] [PubMed] [Google Scholar]
- Marques P, Piqueras L, Sanz MJ (2021). An updated overview of e-cigarette impact on human health. Respir Res; 22(1), 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MS, East KA, Brose LS, McNeill A, Hitchman SC, Partos TR (2021). The effectiveness of using e-cigarettes for quitting smoking compared to other cessation methods among adults in the United Kingdom. Addiction 116(10), 2825–2836. 10.1111/add.15474 [DOI] [PubMed] [Google Scholar]
- McKeganey N, Dickson T (2017). Why Don't More Smokers Switch to Using E-Cigarettes: The Views of Confirmed Smokers. Int J Environ Res Public Health 14(6), 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P (2014). Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev (12), CD010216. [DOI] [PubMed] [Google Scholar]
- Morphett K, Weier M, Borland R, Yong HH, Gartner C (2019). Barriers and facilitators to switching from smoking to vaping: Advice from vapers. Drug Alcohol Rev 38(3), 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CJ, MacLehose RF (2014). Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol 43(3), 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers Smith K, Phillips-Waller A, Pesola F, McRobbie H, Przulj D, Orzol M, Hajek P (2021). E-cigarettes versus nicotine replacement treatment as harm reduction interventions for smokers who find quitting difficult: randomized controlled trial. Addiction 10.1111/add.15628. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (NASEM); Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems; Kathleen Stratton, Leslie Y. Kwan, and David L. Eaton, Editors, 2018. Public Health Consequences of E-Cigarettes. Retrieved September 24, 2021, from https://www.nap.edu/catalog/24952/public-health-consequences-of-e-cigarettes [Google Scholar]
- Newcombe KV, Dobbs PD, Oehlers JS, Dunlap CM, Cheney MK (2021). College Students' Reasons for Using JUULs. Am J Health Promot 35(6), 835–840. [DOI] [PubMed] [Google Scholar]
- Palmer AM, Smith TT, Nahhas GJ, Rojewski AM, Sanford BT, Carpenter MJ, Toll BA (2021) Interest in Quitting e-Cigarettes Among Adult e-Cigarette Users With and Without Cigarette Smoking History. JAMA Netw Open 4(4):e214146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Cuccia A, Willett J, Zhou Y, Kierstead EC, Czaplicki L, Schillo B, Hair EC, Vallone D, 2019. JUUL use and reasons for initiation among adult tobacco users. Tob Control, 28(6), 681–684. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Benmarhnia T, Chen R, White M, Abrams DB, Ambrose BK, Blanco C, Borek N, Choi K, Coleman B, Compton WM, Cummings KM, Delnevo CD, Elton-Marshall T, Goniewicz ML, Gravely S, Fong GT, Hatsukami D, Henrie J, … Messer K (2020). Role of e-cigarettes and Pharmacotherapy during attempts to Quit Cigarette smoking: The PATH Study 2013-16. PLoS One 15(9), e0237938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonavicius E, McNeill A, Arnott D, Brose LS (2017). What factors are associated with current smokers using or stopping e-cigarette use?. Drug Alcohol Depend 173, 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Hrywna M, Wackowski OA, Delnevo CD, Jane Lewis M, Steinberg MB (2017). Knowledge, recommendation, and beliefs of e-cigarettes among physicians involved in tobacco cessation: A qualitative study. Prev Med Rep 8, 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson ME, 2015. (2015). Using longitudinal complex survey data. Annual Review of Statistics and Its Application 2(1), 305–320. [Google Scholar]
- Thompson ME, Fong GT, Boudreau C, Driezen P, Li G, Gravely S, Cummings KM, Heckman BW, O'Connor R, Thrasher JF, Nahhas G, Borland R, Yong HH, McNeill A, Hitchman SC, Quah A (2019). Methods of the ITC Four Country Smoking and Vaping Survey, wave 1 (2016). Addiction 114 Suppl 1(Suppl 1), 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Health and Human Services (US HHS). (2020). Smoking Cessation. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- University of North Carolina Health Care System (2016). Doctors shouldn't routinely recommend e-cigarettes to smokers. ScienceDaily. Retrieved September 23, 2021, from https://www.sciencedaily.com/releases/2016/07/160712073913.htm [Google Scholar]
- Van Gucht D, Baeyens F (2016). Health professionals in Flanders perceive the potential health risks of vaping as lower than those of smoking but do not recommend using e-cigarettes to their Smoking patients. Harm Reduct J 13(1), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrevala T, Coyle A, Walker V, Cabrera Torres J, Ordoña I, Rahman P (2017). A good method of quitting smoking or just an alternative to smoking? Comparative evaluations of e-cigarette and traditional cigarette usage by dual users. Health Psychol Open 4(1), 2055102916684648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, Leavens EL, Tackett AP, Molina N, Queimado L (2017). Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob Control, 26(e1), e23–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RJ, Bhadriraju S, Glantz SA (2021). E-Cigarette Use and Adult Cigarette Smoking Cessation: A Meta-Analysis. Am J Public Health, 111(2):230–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner KE (2019). How to Think-Not Feel-about Tobacco Harm Reduction. Nic Tob Res 21(10), 1299–1309. [DOI] [PubMed] [Google Scholar]
- Wilson S, Partos T, McNeill A, Brose LS (2019). Harm perceptions of e-cigarettes and other nicotine products in a UK sample. Addiction 114(5), 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO). (2021). WHO report on the global tobacco epidemic 2021: addressing new and emerging products. Geneva: World Health Organization. Licence: CC BY-NC-SA 3.0 IGO. file:///C:/Users/smgravel/Downloads/9789240032095-eng%20(2).pdf [Google Scholar]
- Yingst JM, Foulds J, Veldheer S, Hrabovsky S, Trushin N, Eissenberg TT, Williams J, Richie JP, Nichols TT, Wilson SJ, Hobkirk AL (2019). Nicotine absorption during electronic cigarette use among regular users. PloS One, 14(7), e0220300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong HH, Borland R, Cummings KM, Gravely S, Thrasher JF, McNeill A, Hitchman S, Greenhalgh E, Thompson ME, Fong GT (2019). Reasons for regular vaping and for its discontinuation among smokers and recent ex-smokers: findings from the 2016 ITC Four Country Smoking and Vaping Survey. Addiction 114 Suppl 1(Suppl 1), 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong HH, Hitchman SC, Cummings KM, Borland R, Gravely S, McNeill A, Fong GT (2017). Does the Regulatory Environment for E-Cigarettes Influence the Effectiveness of E-Cigarettes for Smoking Cessation?: Longitudinal Findings from the ITC Four Country Survey. Nic Tob Res 19(11), 1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong HH, Karmakar C, Motin MA, Borland R, Elton-Marshall T, Cummings KM, Fong GT, Thompson ME (2021). Identifying factors that conjointly influence nicotine vaping product relative harm perception among smokers and recent ex-smokers: Findings from the 2016 ITC Four Country Smoking and Vaping Survey. Drug Alcohol Depend 218, 108370. [DOI] [PMC free article] [PubMed] [Google Scholar]