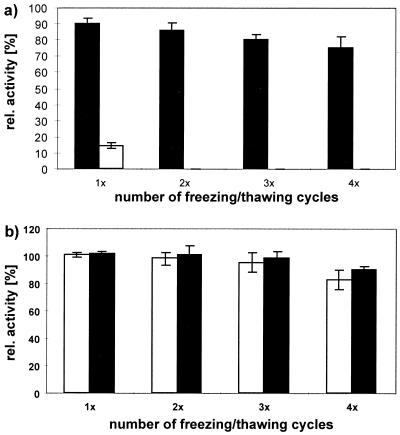
FIG. 6.
Cryoprotection and stabilization of 1 μg of CD30-binding proteins/ml by compatible solutes were measured as relative binding activity by a CD30 receptor ELISA. Specifically bound Ki-4(scFv)-ETA′ was detected after incubation with the anti-ETA′ MAb TC-1 followed by alkaline-phosphatase-conjugated anti-mouse IgG, and bound MAb Ki-4 was detected by alkaline-phosphatase-conjugated anti-mouse IgG. Converted phosphatase substrate (o-phenylenediamine-dihydrochloride) was measured as absorbance at 405 nm. (a) Relative binding activity of Ki-4(scFv)-ETA′ (open bars) compared to that of MAb Ki-4 (solid bars) after several rounds of freeze-thawing. (b) Relative binding activities of Ki-4(scFv)-ETA′ (open bars) and MAb Ki-4 (solid bars) in the presence of 1 M hydroxyectoine.