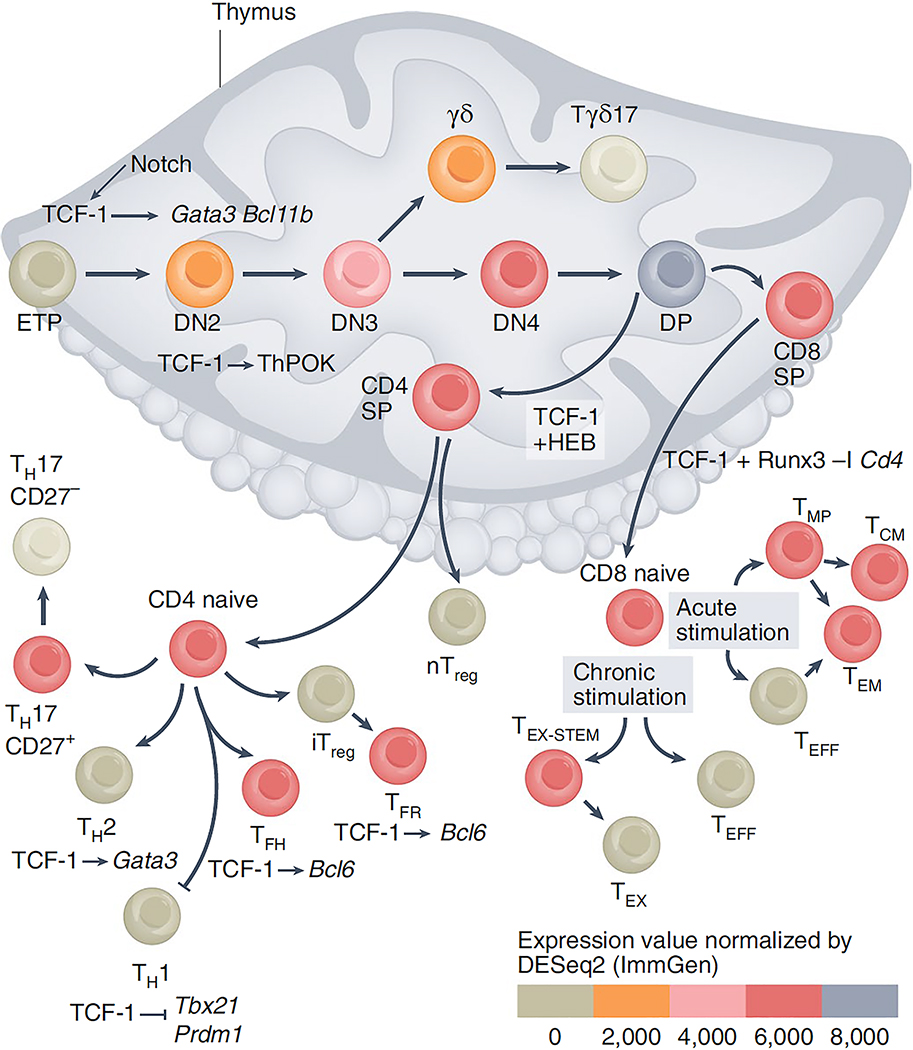
Figure 1: T cell development and the stages of TCF-1 implication.
Notch signaling upregulates Tcf7 expression in early ETPs 35–39. TCF-1 induces the expression of genes encoding transcription factors critical for T cell specification, including Gata3 and Bcl11b. The levels of TCF-1 increases progressively up to the CD4+CD8+ (DP) stage. In CD4+CD8+ (DP) thymocytes TCF-1 cooperates with HEB to define their epigenetic landscape and trasncription profile 44. Following thymic selection the DP cells become either CD4+ or CD8+ SP cells. TCF-1 fosters the CD4+ T cell fate by promoting Zbtb7b (TH-POK) expression, and although TCF-1 is not required for commitment to the CD8+ T cell lineage it ensures CD8+ T cell stability by cooperating with RUNX3 to suppress Cd4 gene expression 48. TCF-1 also suppresses Rorc (RORγT) and Il17 expression in DP thymocytes preventing their conversion to CD4+ TH17 and CD8+ TC17 cells 50,113. After thymic egress CD4+ T cells differentiate into TH subsets. TCF-1 promotes TFH differentiation by inducing Bcl6 and supressing Prdm1 (BLIMP-1) expression and limits TH1 differentiation by suppressing Tbx21 (T-bet) expression 63–65. TCF-1 promotes TH2 differentiation by directly upregulating Gata3 59. TCF-1 is expressed by stem cell-like CD27+ TH17 cells, which persist in models of MS 54. In TREG cell subsets, TCF-1 cooperates with Foxp3 to limit expression of T cell activation and TH17 differentiation genes. It also enhances Bcl6 expression to promote TFR differentiation. CD8+ T cells are critical for defense against acute and chronic viral infections and cancers. In acute viral infection TCF-1 is important in driving the development of TMP and TMEM cells. Both in acute and chronic LCMV infection downregulation of TCF-1 is needed for the differentiation of TEFF and TEX cells. In chronic viral infections and cancer, TCF-1 and BCL-6 support the differentiation and maintenance of TEX-STEM cells that express PD-1 and can respond to anti-PD-1 therapy.