Abstract
Background
Vancouver’s Downtown Eastside (DTES) faces the interrelated challenges of poverty, homelessness, mental health, addiction, and medical issues such as hepatitis C virus (HCV). This study evaluates a new model of engagement with people who inject drugs (PWID) in the DTES.
Methods
Our centre has developed the community pop-up clinic (CPC) to engage vulnerable populations such as PWID. Rapid HCV testing is offered using the OraQuick saliva assay. If a test is positive, immediate medical consultation and an incentivized clinic appointment are offered. At this appointment, an HCV treatment plan is developed, along with a plan for engagement in multidisciplinary care.
Results
In 12 months, 1,283 OraQuick tests were performed at 44 CPCs; 21% of individuals were found to be positive for HCV (68% of whom were PWID). Of individuals positive for HCV antibodies who consulted with the on-site doctor, 50% engaged in care in our clinic—61% of whom have initiated interferon-free directly acting antiviral (DAA) HCV therapy with 100% cured of HCV (per protocol). Individuals who did not engage in care were significantly more likely to be homeless (P < .0001).
Conclusion
CPCs paired with a multidisciplinary model of care address the needs of vulnerable populations such as PWID, particularly in the management of HCV with interferon-free DAA therapies.
Keywords: community outreach, interferon-free DAA HCV therapy, marginalized populations, opioid epidemic
Introduction
An estimated 71 million people globally live with chronic hepatitis C virus (HCV) infection (1, 2). However, HCV treatment programs for people who inject drugs (PWID) have not been part of the response to this epidemic, and fewer than 10% of these individuals receive treatment for HCV infection (3). This low uptake of HCV treatment is associated with barriers such as lack of follow-up on appointments, issues of medical or psychiatric comorbidity, and ongoing substance use (4). HCV affects more than 251,990 individuals in Canada and approximately 60,000 British Columbians (5). On a micro scale, Vancouver’s Downtown Eastside (DTES) is noted for its high-risk populations, with complex interrelated challenges including the previously noted barriers (6,7). This neighbourhood (population 18,500) includes some of the most marginalized and vulnerable people in Canada. Of residents, 60% are male, 10% identify as Indigenous, more than 50% are on social assistance, 10% are homeless, and many more are vulnerably housed (8, 9). More than 80% self-identify as illicit drug users, which makes HCV a great concern given that PWID make up approximately 43% of the HCV antibody–positive population nationwide (10). PWID are also classified as core transmitters of HCV because the mode of transmission is largely injection drug use (11). Given the health and social challenges that people in the DTES face, as well as difficulty in navigating the complex bureaucratic structures necessary to access services such as housing and disability payments, they have a high degree of disengagement from health care. Poor engagement in care has been associated with poor health outcomes, which also include increased mortality and transmission of infectious diseases in the community (12, 13). This population has faced a deadly opioid epidemic, with 1,628 opiate overdose deaths occurring in the same time period as this study (August 2016–August 2017); fentanyl was the cause of death in 83% of those cases (14). In addition, 87% of deaths occurred indoors, which highlights the need for programs and initiatives to engage individuals outside of their homes (14).
The advent of interferon-free direct-acting antiviral (DAA) therapies is transforming HCV into an easily curable disease (15). Increased HCV detection, combined with the availability of simple, well-tolerated treatment regimens, has been shown to result in high adherence and sustained virologic response (SVR) rates of more than 95% (16). In the population of PWID, these therapies are equally as effective and their simplicity and efficacy increase rates of engagement and follow-up (17). However, PWID are often excluded from receiving HCV therapy because of concerns about reinfection, treatment adherence, and effectiveness (18). Conversely, multidisciplinary models of care have been shown to be effective in treating HCV and other health and social issues in the population of PWID, with relatively simple programs that can be established to engage PWID in care (19). Furthermore, a model that successfully engages PWID in care can lead to high treatment uptake and a reduction in cases of reinfection (20).
To meet the World Health Organization’s (WHO’s) goal of eliminating HCV as a public health concern by 2030, target populations such as PWID need to be engaged and maintained in care in a multidisciplinary setting (21). To do so, novel models of outreach and engagement need to be explored. This study outlines the efficacy of community pop-up clinics (CPCs) in engaging PWID in HCV care and initiating interferon-free DAA therapies in a multidisciplinary setting. Moreover, this model of engagement could address this population’s addiction-related needs.
Experimental Procedure
CPC
We designed a novel community-based approach to identify inner-city residents infected with HCV and have designed a model of engagement in care, the CPC model, as a prelude to the initiation of HCV therapy. CPCs are held at specific sites in the DTES and are designed to evaluate as many as 30 adults over a 3-hour period. Sites in the DTES were selected on the basis of their client population, operation hours, and location. Eligible participants were required to provide informed consent and completed a demographic questionnaire. They also signed a release of medical information. The questionnaire was self-administered, but if participants required assistance, trained research associates were available on site. After the questionnaires were completed, participants were tested using the OraQuick HCV Rapid Antibody Test (OraSure Technologies Inc., Bethlehem, PA; sensitivity and specificity >97%) (22). This is a single-use oral swab immunoassay kit to measure the presence of HCV antibodies in a qualitative manner. All participants received a $10 gift card for completing testing and receiving the results, regardless of whether they chose to consult the physician. Participants were given the results of their test in a confidential setting. Participants who tested positive for HCV antibodies were consulted by an infectious disease specialist or nurse on site. Before the consultation, BC Provincial Laboratory databases were reviewed (with the participants’ consent) to deduce whether participants had ever completed HCV testing or was currently engaged in care with another doctor. If participants had a long history of HCV testing with a doctor, they were considered to be already engaged in care. During the consultation, participants were also given the option to make an appointment with a physician at our centre, the Vancouver Infectious Diseases Centre (VIDC). Through this on-site consultation, the physician would confirm whether the participant was currently engaged in care with another physician. Those who scheduled an appointment were given a meal voucher ($10 value) that they could redeem during their visit. Figure 1 provides a flowchart for a typical CPC. Data were gathered over 12 months between August 2016 and August 2017.
Figure 1:
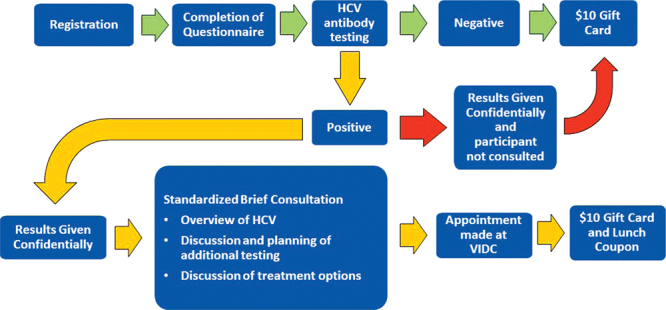
A typical community pop-up clinic flowchart
HCV = Hepatitis C virus; VIDC = Vancouver Infectious Diseases Centre
Appointments and HCV assessment
CPCs were generally held on Friday afternoons, and all HCV-positive participants were given appointments to see an infectious disease specialist on Monday or Tuesday of the following week. Weekly follow-up appointments are made for the next two weeks after the first appointment. If patients attended their first appointment and one other appointment before initiating HCV therapy, they were considered engaged in care. During the first appointment, bloodwork was completed to confirm an active HCV infection, fibrosis was assessed, and patients were assisted with government forms to properly access various social services (welfare, nutrition supplements, temporary housing). Although monetary incentivization for follow-up visits was not provided outside of the first clinic visit, integration into the multidisciplinary model was used to maintain patient engagement. Patients were initiated on HCV therapy as soon as possible. The specific regimen and treatment duration were decided on by the infectious disease specialist, taking into consideration HCV genotype, viral load, liver enzymes, and fibrosis, along with other factors.
Primary and secondary endpoints
The primary endpoint for this study was the engagement of a CPC-recruited patient in multidisciplinary care, as defined by attending two appointments made after contact at CPC in a span of 4 weeks. Secondary endpoints included HCV therapy initiation, achievement of undetectable HCV 12 or more weeks after the end of treatment (HCV cure), and maintenance in post-SVR follow-up.
Multidisciplinary model
All patients at VIDC have access to comprehensive multidisciplinary care with nursing, medical care (including infectious diseases specialty care), and logistic support, which we refer to as the ‘four-legged chair’ model. This model addresses psychiatric, addiction-related, social, and medical needs in an integrated manner. Patients have access to weekly educational support groups, as well as snacks, over-the-counter medications, and daily vitamin supplements, as required. The support groups provide the ability to identify specific medical or social needs; have them addressed through personalized interactions with nurses, clinic staff, and other patients; and receive a free meal. In a typical clinic visit, patients are seen by the physician and receive other support services as needed. CPC patients who attended their appointments were offered a meal if they presented the meal coupon that was provided to them at the CPC. Post-SVR, patients were followed up through standard of care, and attempts were made to follow up with patients every 6 months to monitor HCV reinfection and other comorbidities. Active injection drug users were followed up every 3 months. Patients also had the option to drop in anytime they wanted. Post-SVR engagement is defined as attending their latest appointment post-SVR.
Documentation of overdoses
Overdoses in the engaged population were confirmed through self-reporting during treatment visits and reports provided to VIDC after hospital visits.
Ethics
This study and patient data collection were approved by Chesapeake Institutional Review Board Ethic Board and is annually renewed, as required.
Statistical analysis
All data management and analyses were performed using IBM SPSS version 25.0 (IBM Corp., Armonk, NY). Descriptive statistics were calculated for the demographic variables. A Z-test was used to evaluate specific variables such as homelessness, injection drug use, unemployment, and Indigenous origins in association with disengagement. SVR rate was calculated through per-protocol analysis (excluding those who were lost to follow-up and those who discontinued treatment) and intention-to-treat (ITT) SVR analysis (which included all individuals who received medication). The prevalence of PWID in our HCV antibody–positive cohort was compared with the national value by means of a Z-test.
Results
Patient characteristics
In a 12-month period, 1,283 OraQuick tests were performed at 44 CPCs (median of 29 tests per CPC). The characteristics of this patient population are outlined in Table 1. The mean age was 49 years, 906 (71%) were male, 435 (34%) self-identified as Indigenous, 575 (45%) lived alone, 354 (28%) were homeless, 1,125 (88%) were unemployed, and 942 (73%) self-reported as remote PWID (had not injected drugs in the past 6 months) or active PWID (injected drugs in the past 6 months)
Table 1:
Characteristics of individuals tested at CPC (August 2016–August 2017)
| Variable (N = 1,283) | No.* (%) |
|---|---|
| Mean age | 49 (—) |
| Male | 906 (71) |
| HCV antibody positive | 274 (21) |
| Remote or active PWID† | 188 (69) |
| Indigenous | 435 (34) |
| Lived alone | 575 (45) |
| Homeless | 354 (28) |
| Not working | 1,125 (88) |
| Remote or active PWID | 942 (73) |
* Unless otherwise indicated
† P < .05 compared with nationwide prevalence Active = injected <6 months ago; HCV = hepatitis C virus; PWID = people who inject drugs; Remote = injected >6 months ago
CPC cascade of care and engagement
Of 1,283 tests performed at CPCs, 274 (21%) were found to be HCV antibody positive, 188 (69%) of whom were PWID. Within this subset, 208 patients (76%) chose to consult with the on-site infectious disease specialist. By accessing BC Provincial Laboratory databases and through CPC consultations with the specialist, we found that 42 (20%) of the individuals who consulted with the doctor were already engaged in care elsewhere and were receiving adequate care (eg, receiving HCV treatment or on track to receive treatment). Excluding patients already engaged in care elsewhere, of 166 individuals who were consulted and disengaged from care, 83 (50%) were engaged in care at VIDC. Of the 83 patients who did not engage in care, 56 (67%) were PWID, 44 (53%) were homeless, 72 (87%) were unemployed, and 30 (36%) were Indigenous. Compared with the general CPC study population, individuals who did not engage in care were significantly more likely to be homeless (P < .0001). These data are summarized in Figure 2, Figure 3, and Table 2.
Figure 2:
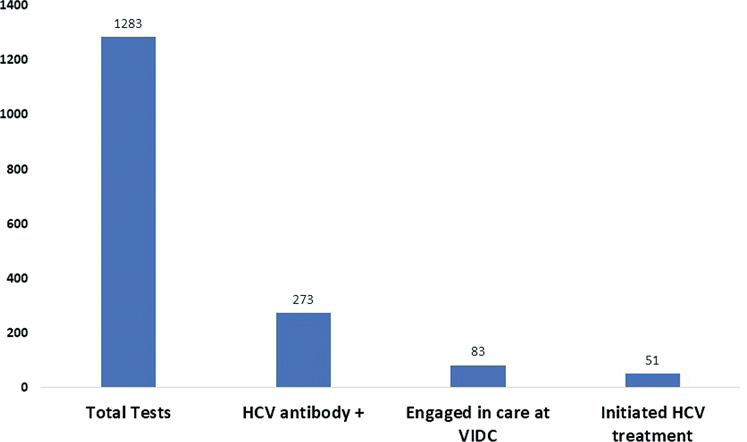
Cascade of care for individuals found to be HCV antibody positive at CPCs (August 2016–August 2017)
CPCs = Community pop-up clinics; HCV = hepatitis C virus; VIDC = Vancouver Infectious Diseases Centre
Figure 3:
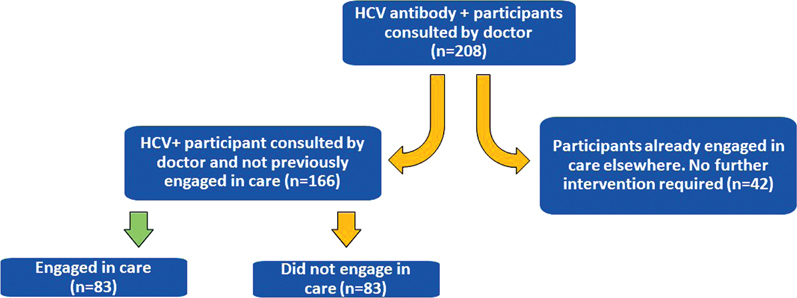
Patient engagement (August 2016–August 2017)
HCV = hepatitis C virus
Table 2:
Factors associated with disengagement (August 2016–August 2017)
| Variable | No. in disengaged population | % (n/83) | P value* (Z test) |
|---|---|---|---|
| PWID | 53 | 64 | .0753 |
| Homeless | 44 | 53 | <.0001 |
| Unemployed | 72 | 87 | .7863 |
| Indigenous | 30 | 36 | .7096 |
* Compared with the general community pop-up clinic population
PWID = People who inject drugs
HCV treatment and post-SVR follow-up
Of the 83 individuals who were successfully engaged in care, 51 had initiated interferon-free DAA HCV therapy. In this cohort, the mean age was 52 years, 42 (82%) were male, and 32 (63%) actively used drugs (had injected in the past 6 months). There were two (4%) opioid overdoses while individuals were engaged in care and no opioid overdose deaths. The most prevalent HCV genotype was 1, affecting 34 (67%) individuals. Eighteen (35%) patients were still on therapy, 2 (4%) discontinued treatment, and 3 (6%) were lost to follow-up. All losses to follow-up occurred between the end of treatment and the SVR time point, 12 weeks posttreatment. Twenty-eight individuals achieved SVR with no relapses, giving a per-protocol SVR rate of 100% and an ITT SVR rate of 85%. In the cohort who achieved SVR, the mean person-days of follow-up was 256 days, with no incidences of reinfection. Patient demographics and treatment statistics are presented in Table 3 and 4.
Table 3:
Demographics of patients initiated on HCV interferon-free DAA therapy (August 2016–August 2017)
| Patients (n = 51) | No.* (%) |
|---|---|
| Mean age, years | 52 |
| Male | 42 (82) |
| Active drug use | 32 (63) |
| Heroin | 22 (69) |
| Cocaine | 17 (53) |
| Other | 11 (34) |
| Opioid overdose | 2 (4) |
| Opioid overdose deaths | 0 (—) |
| HCV genotype | |
| 1a | 33 (65) |
| 1b | 1 (2) |
| 2 | 5 (10) |
| 3 | 12 (23) |
*Unless otherwise specified
DAA = Directly acting antiviral; HCV = Hepatitis C virus
Table 4:
Treatment statistics (August 2016–August 2017)
| Variable | No.* (%) |
|---|---|
| CPC all-oral cohort (n = 51) | |
| Still on therapy | 18 (35) |
| SOF/VEL | 14 (82) |
| PRoD + RBV | 1 (5) |
| ELB/GRAZ | 3 (17) |
| Patients in whom SVR was evaluable (n = 33) | |
| Discontinued therapy | 2 (4) |
| PRoD + RBV | 1 (50) |
| VEL+SOF | 1 (50) |
| Lost to follow-up | 3 (6) |
| ELB/GRAZ | 2 (67) |
| PRoD + RBV | 1 (33) |
| Achieved SVR | 28 (55) |
| ELB/GRAZ | 4 (14) |
| SOF/LDV | 2 (7) |
| SOF/VEL | 5 (18) |
| SOF/VEL/VOX | 1 (4) |
| G/P | 1 (4) |
| PRoD + RBV | 12 (43) |
| Other | 3 (10) |
| Relapse | 0 (—) |
| SVR rate (ITT) | 28/33 (85) |
| SVR rate (per protocol) | 28/28 (100) |
| Mean person-days of follow-up in SVR cohort | 256 (—) |
| Confirmed cases of reinfection | 0 (—) |
* Unless otherwise noted
CPC = Community pop-up clinic; ELB = Elbasvir; G = Gleacaprevir; GRAZ = Grazoprevir; ITT = Intention to treat; LDV = Ledispavir; P = Pibrentasvir; PRoD = Paritaprevir/ritonavir/ombitasvir + dasabuvir; RBV = Ribavirin; SOF = Sofosbuvir; SVR = Sustained virologic response; VEL = Velpatisvir; Vox = Voxelaprevir
Discussion
Despite the existence of effective therapies for the cure of HCV, marginalized populations have been systematically excluded from receiving treatment or have been unable to acquire treatment as a result of social and bureaucratic barriers (23). We describe a novel model of community outreach to engage vulnerable populations such as PWID in HCV treatment and to maintain these populations in multidisciplinary care.
Over 12 months, 1,283 Oraquick tests were performed in Vancouver’s DTES, demonstrating an HCV prevalence of 21%; 68% of subjects were past or current injection drug users. The prevalence of PWID in the population is significantly higher than the national value (P < .0001) (10). This underscores the critical need to address the requirements of this DTES population quickly to prevent the transmission of HCV via core transmitters. A large proportion of the population self-identified as unemployed (88%), which indicates widespread income assistance dependence in this population, a factor that has previously been linked to poor health outcomes (24). Furthermore, income assistance laws in British Columbia designate that earning more than $2,000 per month (including welfare assistance) disqualifies an individual from receiving income assistance (25). In a city in which the average rent price hovers around $2,000 per month, this level of income assistance only serves to perpetuate the marginalization of this population (26).
After testing at CPC, patients who were positive for HCV (n = 274) were encouraged to consult with an infectious disease specialist on site, and 76% chose to do so. This 24% loss speaks to the vulnerability of this population. Anecdotally, many patients who did not want to speak to the physician were late for work, had another appointment, or simply wanted the gift card for getting tested. We therefore need to develop different engagement strategies for people who test positive for HCV because, ideally, more than 95% of these people should consult an infectious disease specialist to at least gain some knowledge about the disease and its treatment. Monetary incentivization of consultation could perhaps be an avenue worth pursuing for people who are unwilling to speak to the on-site physician. CPC patients who attended their first clinic visit and brought their meal voucher were provided with a complementary meal. Of the patients who attended the clinic, 100% (n = 83) redeemed this voucher, suggesting that this model of incentivization is an effective way to encourage clinic attendance. Of the population who did not engage in care, a significant portion were homeless. In similar HCV–HIV coinfected populations, homelessness was found to be a predictor of nonengagement (27). Competing priorities could be keeping these individuals disengaged from care, especially because HCV has been classified as a silent killer that does not manifest symptomatically for the majority of the disease course, leaving those infected feeling healthy and unconcerned about treatment (28).
In the era of interferon-free DAA regimens, excluding PWID from receiving treatment because of the fear of nonengagement and reinfection is no longer valid reasoning. In our multidisciplinary setting, 51 of the 83 individuals engaged in care initiated treatment, with the remaining on track to initiate treatment in the near future. These 32 individuals completed screening to initiate treatment and are waiting for HCV medication funding through the BC government or through pharmaceutical companies’ compassionate care programs. This cohort includes active drug users, despite older guidelines advocating for abstinence periods for such individuals (18). In this incredibly vulnerable cohort, there were only two opioid overdoses with no deaths while patients were engaged with us. In the same time frame as this study, there were 1,628 opioid overdose deaths in a population of approximately 14,800 PWID, giving an overdose prevalence of 10% in the DTES (8, 14). Comparatively, an overdose prevalence of 4% in our cohort could be related to the efficacy of CPCs and the multidisciplinary care PWID received. With 87% of opioid-related deaths occurring indoors, it is imperative to encourage individuals to leave their residences, and this multidisciplinary model encourages such interaction by providing not only HCV care but other services related to this population’s comorbidities.
In this treatment cohort, two discontinuations occurred as a result of social issues, and 3 individuals were lost to follow-up. With interferon-free DAA regimens, 28 individuals reached the SVR time point and achieved an HCV cure (no viral relapse), giving a per-protocol SVR rate of 100%. In the ITT population (which includes those who discontinued treatment and those lost to follow-up), the SVR rate was 85%. It is worth noting that this is a rolling cohort, and the ITT SVR rate may be artificially deflated by including the additional patients who discontinued therapy early. Despite this, and given the nature of this population, these data are promising in that they confirm the results of clinical trials such as SIMPLIFY in a real-world setting (29). Other real-world studies have found similar results with active or non–active drug users, further advocating for the expansion of HCV therapy for injection drug users (30, 31). However, patient recruitment and retention in a multidisciplinary model is not described in these real-world studies, which could result in higher rates of reinfection in such a high-risk population (32). Of 274 HCV antibody–positive individuals we encountered, treatment uptake was 19% (n = 51), which is higher than in other large cohort studies (3). Considering that all losses to follow-up occurred before the SVR time point, using this model we were able to achieve a mean follow-up of 256 days post-SVR with no cases of reinfection, further confirming the efficacy of CPCs and a multidisciplinary follow-up model in diagnosing, treating, and preventing subsequent HCV infections.
This study has inherent limitations. Given the nature of this population, some individuals were tested more than once at different CPCs, resulting in duplicate data. Although the team attempted to mitigate duplicate tests, participants may have falsified names to obtain the incentive provided with testing. Recently, the CPC team has brought a portable, secured copy of the database to check whether participants have previously been tested in hopes of eliminating duplicates. Furthermore, hospitalization and self-reports were used to document overdoses, which may result in an underestimation of overdoses in this population. Although gift cards are provided simply for getting tested and receiving results, some participants only wanted to receive a gift card and had no interest in speaking to a physician if they were HCV antibody positive, outlining another limitation of this model.
Conclusion
As we near almost-perfect SVR rates in an era of interferon-free DAA HCV therapy, populations of core transmitters cannot be excluded if we want to reach WHO’s target of eliminating HCV by 2030. The combined model of CPCs and a multidisciplinary system is a novel approach to testing, diagnosing, and engaging marginalized individuals living in Vancouver’s DTES as well as to initiate treatment once individuals are engaged in care. Homelessness was significantly associated with participant nonengagement, demonstrating the need for targeted intervention with this subgroup. With this model, opioid overdoses could be minimized, despite the current high rate of opioid deaths in Vancouver. Outside of a micro-elimination setting such as the DTES, this model can be expanded to other areas that have a high proportion of injection drug users to reach the goals set by the WHO.
Acknowledgments
Acknowledgements
This article is part of a special topic series commissioned by the Canadian Network on Hepatitis C (CanHepC). CanHepC is funded by a joint initiative of the Canadian Institutes of Health Research (NHC-142832) and the Public Health Agency of Canada. The authors thank Vancouver Infectious Diseases Centre patients and staff for their contributions to this work.
Ethics Approval:
N/A
Informed Consent:
N/A
Registry and Registration No. of The Study/Trial:
N/A
Funding
This work was supported in part by a research grant from the Investigator Initiated Studies Program of Merck Canada Inc. The opinions expressed in this article are those of the authors and do not necessarily represent those of Merck Canada Inc. or its affiliates or related companies. Merck & Co. had no role in in the analysis or interpretation of the results.
Disclosures
Dr. Conway reports grants, honouraria, personal fees, and nonfinancial support from Merck & Co, Abbvie, Gilead Sciences, and Viiv. Mr. Alimohammadi reports personal fees and nonfinancial support from Merck & Co., Abbvie, and Gilead Sciences. Dr. Truong reports personal fees and nonfinancial support from Merck & Co. Ms. Holeksa reports grants from Merck & Co., during the conduct of the study. Ms Parsons reports grants from Merck & Co., during the conduct of the study. Ms Yung reports grants from Merck & Co., during the conduct of the study.
Peer Review:
This article has been peer reviewed.
Funding Statement
This work was supported in part by a research grant from the Investigator Initiated Studies Program of Merck Canada Inc.
References
- 1.Blach S,Zeuzem S,Manns M, et al.; Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–76. 10.1016/S2468-1253(16)30181-9. Medline: [DOI] [PubMed] [Google Scholar]
- 2.Degenhardt L,Peacock A,Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5(12):e1192–207. 10.1016/S2214-109X(17)30375-3. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alavi M,Raffa JD,Deans GD, et al. Continued low uptake of treatment for hepatitis C virus infection in a large community-based cohort of inner city residents. Liver Int. 2014;34:1198–206. 10.1111/liv.12370. Medline: [DOI] [PubMed] [Google Scholar]
- 4.Mehta SH,Genberg BL,Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33:126–33. 10.1007/s10900-007-9083-3. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers RP,Krajden M,Bilodeau M, et al. Burden of disease and cost of chronic hepatitis C infection in Canada. Can J Gastroenterol Hepatol. 2014;28:243–50. 10.1155/2014/317623. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen CK,Hudak PL,Hwang SW. Homeless people’s perceptions of welcomeness and unwelcomeness in healthcare encounters. J Gen Intern Med. 2007;22:1011–7. 10.1007/s11606-007-0183-7. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajabiun S,Mallinson RK,McCoy K, et al. ‘Getting me back on track’: the role of outreach interventions in engaging and retaining people living with HIV/AIDS in medical care. AIDS Patient Care STDS. 2007;21(Suppl 1):S20–9. 10.1089/apc.2007.9990. Medline: [DOI] [PubMed] [Google Scholar]
- 8. City of Vancouver Community Services—Social Development, Social Policy, City of Vancouver Planning and Development Services—Central Area Planning, Downtown Eastside Group, Vancouver, Canada. Downtown Eastside Local Area Profile; 2013. http://vancouver.ca/files/cov/profile-dtes-local-area-2013.pdf (January 5, 2018). [Google Scholar]
- 9.Thomson M. Vancouver Homeless Count 2016. 2016. http://vancouver.ca/files/cov/homeless-count-2016-report.pdf (January 5, 2018).
- 10.Trubnikov M,Yan P,Archibald C. Estimated prevalence of hepatitis C virus infection in Canada, 2011. CCDR. 2014; 40–19:421–9. http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/14vol40/dr-rm40-19/surveillance-b-eng.php (January 5, 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krajden M,Montaner JSG. The curability of hepatitis C: Implications for a public health response. BCMJ. 2013;55(5):243. [Google Scholar]
- 12.Giordano TP,Gifford AL,White AC Jr, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–9. 10.1086/516778. Medline: [DOI] [PubMed] [Google Scholar]
- 13.Mugavero MJ,Lin HY,Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48:248–56. 10.1086/595705. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. British Columbia Coroners Service, Vancouver, British Columbia, Canada. Illicit drug overdose deaths in BC January 1, 2007–October 31, 2017. https://www2.gov.bc.ca/assets/gov/public-safety-and-emergency-services/death-investigation/statistical/illicit-drug.pdf (January 4, 2018).
- 15.Kohli A,Shaffer A,Sherman A, et al. Treatment of hepatitis C: a systematic review. JAMA. 2014;312:631–40. 10.1001/jama.2014.7085. Medline: [DOI] [PubMed] [Google Scholar]
- 16.Grebely J,Hajarizadeh B,Dore GJ. Direct-acting antiviral agents for HCV infection affecting people who inject drugs. Nat Rev Gastroenterol Hepatol. 2017;14:641–51. 10.1038/nrgastro.2017.106. Medline: [DOI] [PubMed] [Google Scholar]
- 17.Grebely J,Litwin A,Dore GJ. Addressing reimbursement disparities for direct-acting antiviral therapies for hepatitis C virus infection is essential to ensure access for all. J Viral Hepat. 2016;23:664–6. 10.1111/jvh.12550. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffin C,Fung S,Ma M. Management of chronic hepatitis C: Canadian Association for the Study of the Liver consensus guidelines. Can J Gastroenterol. 2012;26:917–38. 10.1155/2012/506819. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grebely J,Genoway K,Khara M, et al. Treatment uptake and outcomes among current and former injection drug users receiving directly observed therapy within a multidisciplinary group model for the treatment of hepatitis C virus infection. Int J Drug Policy. 2007;18:437–43. 10.1016/j.drugpo.2007.01.009. Medline: [DOI] [PubMed] [Google Scholar]
- 20.Alimohammadi A,Singh A,Raycraft T, et al. Reduced HCV recurrent viremia in people who inject drugs (PWID) after treatment induced HCV cure. Int J Curr Advanced Res. 2017;6:2075–7. [Google Scholar]
- 21. World Health Organization. Combating hepatitis B and C to reach elimination by 2030. Geneva: World Health Organization; 2016. http://apps.who.int/iris/bitstream/handle/10665/206453/WHO_HIV_2016.04_eng.pdf;jsessionid=D2ACC5BF329723781D34EDBDFD35BA20?sequence=1(January 5, 2018). [Google Scholar]
- 22.Cha YJ,Park Q,Kang E-S, et al. Performance evaluation of the OraQuick hepatitis C virus rapid antibody test. Ann Lab Med. 2013;33:184–9. 10.3343/alm.2013.33.3.184. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edlin BR,Kresina TF,Raymond DB, et al. Overcoming barriers to prevention, care, and treatment of hepatitis C in illicit drug users. Clin Infect Dis. 2005;40(suppl 5):S276–85. 10.1086/427441. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergqvist K,Yngwe MA,Lundberg O. Understanding the role of welfare state characteristics for health and inequalities—an analytical review. BMC Public Health. 2013;13(1):1234. 10.1186/1471-2458-13-1234. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldsmith P. How to apply for welfare [document on the Internet]. Vancouver: Legal Services Society; 2017. https://www.lss.bc.ca/resources/pdfs/pubs/How-to-Apply-for-Welfare-eng.pdf (January 5, 2018). [Google Scholar]
- 26.Ip S. Average rental cost for one-bedroom apartment in Vancouver is now $2,020. Vancouver Sun [newspaper on the Internet]. 2017. September 15 [cited 2018 January 2]. Available from: http://vancouversun.com/news/local-news/average-rental-cost-for-one-bedroom-apartment-in-vancouver-is-now-2020.
- 27.Cachay ER,Hill L,Wyles D, et al. The hepatitis C cascade of care among HIV infected patients: a call to address ongoing barriers to care. PLoS One. 2014;9(7):e102883. 10.1371/journal.pone.0102883. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirsch KR,Wright TL. “Silent killer” or benign disease? The dilemma of hepatitis C virus outcomes. Hepatology. 2000;31:536–7. 10.1002/hep.510310241. Medline: [DOI] [PubMed] [Google Scholar]
- 29.Conway B,Grebely J,Fraser C, et al. Paritaprevir/ritonavir/ombitasvir, dasabuvir + ribavirin in people with HCV genotype 1 and recent injecting drug use or receiving Ost: D3feat Study. Paper presented at 6th International Symposium on Hepatitis Care in Substance Users; Jersey City, NJ; 2017 September 8. [Google Scholar]
- 30.Norton BL,Fleming J,Bachhuber MA, et al. High HCV cure rates for people who use drugs treated with direct acting antiviral therapy at an urban primary care clinic. Int J Drug Policy. 2017;47:196–201. 10.1016/j.drugpo.2017.07.021. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazhnaya A,Meteliuk A,Barnard T, et al. Implementing and scaling up HCV treatment services for people who inject drugs and other high risk groups in Ukraine: an evaluation of programmatic and treatment outcomes. Int J Drug Policy. 2017;47:187–95. 10.1016/j.drugpo.2017.07.023. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons B,Saleem J,Hill A, et al. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin Infect Dis. 2016;62:683–94. 10.1093/cid/civ948. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]


