Table 2.
Parameters proposed to be related to the metabolite intestinal concentrations, and the parameter distributions used to model variability in possible concentrations of antimicrobially active ceftiofur metabolites in the small and large intestines of cattle treated with ceftiofur parenterally
| Parameter | Definition | Distribution | Data used to derive distribution |
|---|---|---|---|
 |
Rate of ceftiofur metabolites biotic degradation in small and large intestines per hour (possible dependency of β-lactamase production by enteric bacteria on the CE concentration was not included) | Uniform (0·15, 0·25) | [44–45] |
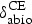 |
Rate of metabolites abiotic degradation in GI tract through hydrolysis | – | Antimicrobial activity of main product is close to parent drug |
 |
Fraction of metabolites excreted in bile | Uniform (0·24, 0·45) | [1] |
 |
Fraction of metabolites adsorbed to the digesta in the lower 2/3 of small intestine | – | Not included in model simulations |
 |
Fraction of metabolites adsorbed to the digesta in large intestine | – | Not included in model simulations |
 |
Fractional digesta flow through the upper 1/3 of small intestine per hour | 0·3237 | [35] |
 |
Fractional digesta flow through the lower 2/3 of small intestine per hour | 0·1619 | [35] |
 |
Fractional digesta flow through large intestine per hour (to defecation) | 0·3030 | [35] |
 |
Volume of digesta in the lower 2/3 of small intestine, litre | Uniform (4, 23) | [38] |
| Vli | Volume of digesta in large intestine, litre | Uniform (6, 22) | [38] |
Deterministic estimates of the dynamics of ceftiofur metabolites in the central circulation published by the drug manufacturer were used in all simulations. Intestinal transit time of ceftiofur metabolites was as for the liquid digesta phase.
