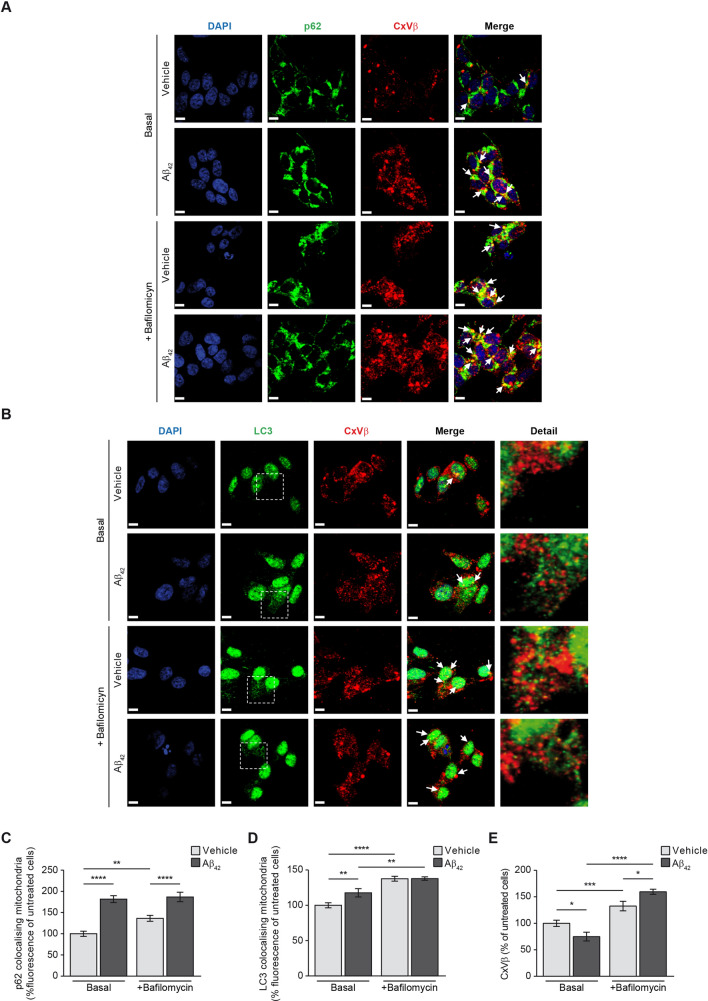
Figure 4.
Aβ increases mitophagy. The effect of Aβ on mitophagy was evaluated in SH-SY5Y cells by analysing the colocalisation of the mitochondria with the main autophagy markers p62 (A and C) and the punctate-like structures of LC3-II (B and D) by immunofluorescence (white arrowheads). Representative images of SH-SY5Y cells treated with or without 1 µM oligomerised Aβ42 for 24 h (Basal). The autophagosome containing mitochondria accumulation was evaluated by addition of the autophagosome—lysosome fusion inhibitor bafilomycin (100 nM). In all cases, the mitochondria were located using the specific antibody against the structural protein beta subunit of mitochondrial Complex V (CxVβ; stained in red). The p62 (A) and punctate-like structures of LC3-II (B) as autophagy markers were located using the specific antibodies (stained in green) and the nuclei were stained with DAPI (blue). Scale bar = 9 µm. (C and D) Histograms show only the colocalisation levels (yellow signal in the images, and arrowheads) between the mitochondria and p62 (C) and between the mitochondria and LC3-II (D) that were estimated analysing the fluorescence in the basal stage and in the presence of bafilomycin (100 nM) with or without 1 µM oligomerised Aβ42 for 24 h. (E) Histogram showing the amount of mitochondria that was estimated by analysing the fluorescence levels of the structural protein CxVβ with or without 1 µM oligomerised Aβ42 for 24 h and in absence (Basal) or presence of bafilomycin (100 nM). Data are expressed as mean ± SEM; n = 5. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Statistical significance was assessed by one-way ANOVA followed by Fisher’s post hoc test for multiple comparisons.