FIGURE 1.
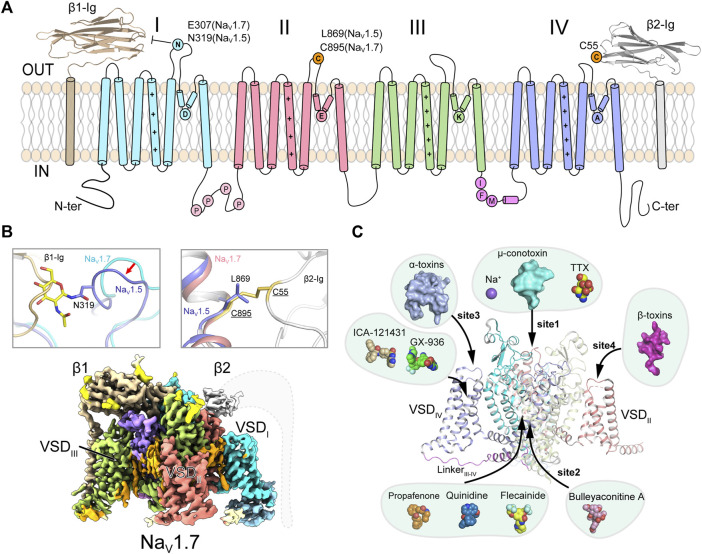
Topology and architecture of mammalian NaV channels. (A) Topology of mammalian NaV channels. DI, DII, DIII, IFM-motif and DIV of α-subunit, β1 and β2 subunit are colored in cyan, red, green, purple, blue, brown and gray, respectively. A unique N-glycosylation site in NaV1.5 and the cysteine residue for linking β2 are highlighted. P represents phosphorylation sites. (B) Subunits assembly of mammalian NaV channels. A sugar moiety of N319 glycosylation and L869 block the binding of β1 and β2 to NaV1.5 (PDB code: 6UZ3), respectively (Upper panels). Cryo-EM map of human NaV1.7/β1/β2 (lower panel, EMDB code: EMD-33292). Dashed lines indicate the invisible region of β2 subunit. (C) Multiple ligand binding sites in mammalian NaV channel α-subunit. Sodium ion and small-molecule modulators shown in spheres, polypeptide toxins shown in surface.