Abstract
The addition of palbociclib (a cyclin-dependent kinase 4/6 inhibitor) to endocrine therapy (ET) has been shown to significantly improve progression-free survival (PFS) and overall survival (OS) among patients with hormone receptor-positive (HR+) advanced breast cancer. The current study presents the local experience of using palbociclib at two cancer centers in Saudi Arabia. Electronic data of patients with metastatic HR+ and human epidermal growth factor receptor 2-negative breast cancer who progressed after prior ET and received at least one cycle of palbociclib plus ET, were retrospectively reviewed. A total of 97 patients were identified, and their data were included in the analysis. The median age of the patients was 55 years. Patients were heavily pretreated in the metastatic setting (55% received systemic chemotherapy and 49% received two or more lines of prior ET). In total, 29 (30%) and 50 (52%) patients achieved an objective response and clinical benefit, respectively. The median follow-up time was 31.0 months [95% confidence interval (CI), 16.9-44.9] and the median PFS time was 16.3 months (95% CI, 11.4-21.2), with 58% of patients remaining progression-free at 12 months. Upon multivariate regression analysis, liver involvement was the only significant independent variable that predicted a greater risk of progression or death (hazard ratio, 2.32; 95% CI, 1.22-4.40; P=0.010). The median OS time was 19.6 months (95% CI, 18.1-20.9), with 12- and 24-month OS rates of 75 and 30%, respectively. Overall, real-world data showed that administration of palbociclib in combination with ET in patients with advanced HR+ breast cancer achieved a favorable outcome that was comparable to that reported in clinical trials.
Keywords: therapy, palbociclib, breast cancer, human epidermal growth factor receptor 2, estrogen receptor, cyclin-dependent kinase 4/6 inhibitor
Introduction
Breast cancer is the most common cancer in women in numerous countries, including developing countries, and remains the foremost cause of cancer-associated mortality in women globally. In Saudi Arabia, the disease accounts for ~30% of cancer cases in women (1).
Of all breast cancer subtypes, hormone-receptor-positive (HR+) breast cancer is the most common, affecting more than 1 million patients annually worldwide (2,3). For several decades, advances in adjuvant endocrine therapy (ET) have significantly improved patient survival. Nevertheless, this subtype remains associated with a considerable risk of recurrence that may occur even after a number of years (4).
It has long been known that cyclin-dependent kinases 4 and 6 (CDK4/6) are crucial promoters of tumor growth in HR+ breast cancer (5-7). More recently, pivotal randomized trials showed that the addition of CDK4/6 inhibitors (palbociclib, ribociclib or abemaciclib) to either aromatase inhibitors in first-line therapy or fulvestrant in the second-line setting for HR+ and human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer improved progression-free survival (PFS) and overall survival (OS) times among premenopausal and postmenopausal women alike (8). It was also shown that CDK4/6 inhibitors demonstrated a favorable effect on quality of life (9,10).
Real-world data in oncology are needed due to the inherent characteristics of clinical trials, which include, but are not limited to, selection bias due to barriers to accessing clinical trials, concerns about a specific group of patients, including patients with limited comorbidities, and the fact that the trials are generally conducted in facilities where investigators have the experience of that specific drug or product (11). Real-world evidence is thus important for assessing clinical trial results across different patient populations (12).
Data about the efficacy of CDK4/6 inhibitors in patients with metastatic HR+/HER2- breast cancer in developing countries are lacking. This dearth of information prompted the current analysis of the local experience of two cancer centers in Saudi Arabia, examining the use of palbociclib combined with appropriate ET for women with HR+/HER2- breast cancer who progressed after at least one prior ET for advanced disease.
Patients and methods
Study setting
The present retrospective study was conducted on data from patients treated between March 2016 and February 2021. The electronic records of women with metastatic breast cancer were collected at two oncology centers, the Princess Noorah Oncology Center (PNOC) (Jeddah, Saudi Arabia) and the International Medical Center (IMC) (Jeddah, Saudi Arabia). Ethical approval was obtained from the Institutional Review Board Committee of King Abdullah International Medical Research Center (Jeddah, Saudi Arabia).
The inclusion and exclusion criteria were as follows: i) Women with metastatic HR+ and ii) human HER2- breast cancer who had received iii) at least one cycle of palbociclib plus ET. Patients with HER-2+ tumors or triple-negative breast cancer were not eligible for inclusion in this study. Only patients who progressed after at least one prior line of ET for advanced disease were included regardless of menopausal status. Patients who had previously received other CDK4/6 inhibitors were excluded from the study.
The following data were exhaustively collected: Patient age, menopausal status, date of initiation of palbociclib, date and disease stage at initial diagnosis, type of adjuvant therapy for early stage disease, date of disease recurrence for patients who initially presented with early stage disease, prior therapy for metastatic or recurrent disease, sites of metastases at the initiation of palbociclib, palbociclib dose reductions, disease response, date of disease progression and date of death or last follow-up.
Additionally, data were retrieved on prior sensitivity to ET, which was defined as either a documented complete response, partial response or stable disease for >24 weeks from at least one previous ET regimen for metastatic disease or at least 24 months of adjuvant ET before documented disease recurrence (13).
Oral palbociclib was administered at 125 mg daily for 21 consecutive days, followed by 7 days off for a 28-day cycle. Reduction in the daily dose of palbociclib due to adverse events was performed according to standard guidelines, first to 100 mg, then to 75 mg and then to 75 mg for 2 weeks on and 2 weeks off (14). The companion ET was either an aromatase inhibitor or a fulvestrant used according to the standard of care. The choice of a particular ET was made based on the previous adjuvant therapy administered or the preference of the physician or patient. For premenopausal women, adequate ovarian function suppression was mandated and obtained by either laparoscopic bilateral oophorectomy or using the luteinizing hormone-releasing hormone agonist goserelin.
Statistical analysis
Disease response was evaluated in patients with measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1(15). For patients with bone-only disease, the response was evaluated using computed tomography or magnetic resonance imaging.
PFS time was defined as the interval between the date recorded at 1-month post-first administration of palbociclib and the date of progression or death from any cause, whichever came first. OS time was defined as the interval between the date recorded at 1-month post-first administration of palbociclib to the date of death from any cause or the date of the last follow-up. Survival functions were computed using the Kaplan-Meier method, and the difference in survival between groups was compared using the log-rank test. The univariate Cox's conditional logistic regression method was used to explore the independent associations of relevant clinicopathological variables and PFS. Only variables with P≤0.1 were included in the multivariate model, with variables with P>0.05 eliminated in a backward selection process. The final model only included variables with P≤0.05. The hazard ratio (HR) and its 95% confidence interval (CI) were reported. All tests were two-sided and P≤0.05 was used to indicate a statistically significant difference. Statistical analyses were performed with the SPSS statistical package (version 25.0; IBM Corp.).
Results
Demographic data
A total of 67 and 30 patients were identified from the PNOC and the IMC, respectively, and were included in the analysis. Table I depicts patient demographics and disease characteristics. Patients were predominantly middle-aged (median age, 55 years) and a number (n=37) had associated comorbidities. The included patients were heavily pretreated for metastatic disease (55% received systemic chemotherapy and 49% received two or more lines of prior ET). Overall, 66% of patients had two or more metastatic sites. Due to adverse events, 29 patients (30%) required a dose reduction of palbociclib. The patients who required a dose reduction experienced a range of adverse reactions, including fever, gastrointestinal disorders, renal/urinary disorders, metabolic disorder/imbalance, nervous system disorders, cardio-respiratory symptoms and anemia. There were 21 (22%) and 7 (7%) occurrences of dose reduction to 100 and 75 mg, respectively. There was no incidence of therapy discontinuation due to adverse events. As initial tumor stage has no value in the metastatic setting, except for the data regarding de novo vs. recurrent disease, disease-free interval, and the number and sites of metastatic disease, the patients with HER-2+ tumors or triple-negative breast cancer were not included in this study, neither was tumor stage data for the analysis of PFS (13,14,16,17).
Table I.
Demographic and clinical characteristics of the patients with breast cancer in the present study.
| Characteristic | Value |
|---|---|
| Total patients, n | 97 |
| Agea, years | |
| Median (95% CI) | 55 (52-57) |
| <50, n (%) | 25(26) |
| ≥50, n (%) | 72(74) |
| Menopausal statusa, n (%) | |
| Perimenopausal | 30(31) |
| Postmenopausal | 67(69) |
| Comorbiditya, n (%) | |
| Hypertension | 14(14) |
| Diabetes | 14(14) |
| Cardiovascular disease | 9(9) |
| Others | 64(66) |
| Stage at initial diagnosis, n (%) | |
| Early/locally advanced | 53(55) |
| Metastatic | 44(45) |
| Hormone receptor status, n (%) | |
| ER-positive | 97(100) |
| PR-positive | 84(87) |
| Adjuvant ET, n (%) | 53(55) |
| Disease-free interval for early diseaseb, n (%) | |
| <12 months | 32(33) |
| 12-24 months | 2(2) |
| >24 months | 19(20) |
| Prior chemotherapya, n (%) | |
| None | 9(9) |
| Neo/adjuvant | 20(21) |
| For metastatic disease | 53(55) |
| Neo/adjuvant/metastatic | 15(15) |
| ET lines for metastatic diseasea, n (%) | |
| One | 49(51) |
| Two | 31(32) |
| Three or more | 17(18) |
| Prior ET sensitivitya, n (%) | |
| Yes | 9(9) |
| No | 77(79) |
| Unknown | 11(11) |
| Metastatic sitesa, n (%) | |
| Bone only | 26(27) |
| Bone | 84(87) |
| Lung/pleura | 42(43) |
| Liver | 56(58) |
| Brain | 18(19) |
| Number of metastatic sitesa, n (%) | |
| One | 33(34) |
| Two | 35(36) |
| Three or more | 29(30) |
| Response to therapya, n (%) | |
| Complete response | 4(4) |
| Partial response | 25(26) |
| Stable disease | 21(22) |
| Progression | 36(37) |
| Not assessable | 11(11) |
aAt palbociclib initiation.
b(n=53). CI, confidence interval; ET, endocrine therapy; ER, estrogen receptor; PR, progesterone receptor.
Objective response rate (ORR)
Of the 86 patients who had measurable, assessable metastatic sites, 30% achieved an ORR (complete and partial response), while 52% attained a clinical benefit (ORR and stable disease) and 37% of patients had progression of the disease. Another 11% of the patients were not accessible for the assessment; those patients had no measurable disease, thus an accurate assessment of response was not possible (Table I). The measurability of the disease was set as per RECIST guidelines (15).
Survival analysis
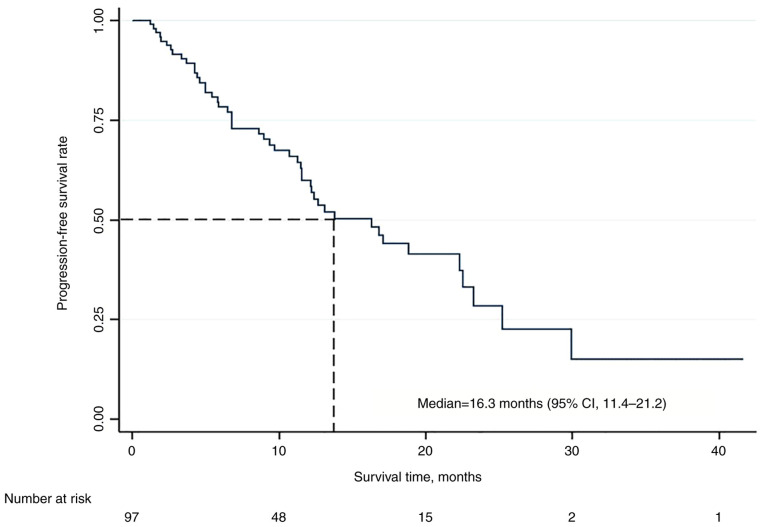
The median follow-up time was 31.0 months (95% CI, 16.9-44.9). The median PFS time was 16.3 months (95% CI, 11.4-21.2) (Fig. 1), with 58% of patients remaining progression-free at 12 months. Univariate analysis of the independent effect of various relevant clinicopathological variables was performed evaluating the following variables: Age, menopausal status, recurrent vs. de novo metastatic disease, the disease-free status, bone-only disease and visceral involvement. Only liver involvement, presence of bone-only disease and involvement of one metastatic site vs. more than one site were chosen to be included in the multivariate model of analysis (Table II).
Figure 1.
Kaplan-Meier survival curve for progression-free survival. CI, confidence interval.
Table II.
Univariate and multivariate analysis of the independent effect of variables on progression-free survival.
| Variables | Univariate analysis, HR (95% CI) | P-value | Multivariate analysis, HR (95% CI) | P-value |
|---|---|---|---|---|
| No. of metastatic sites | ||||
| More than one site | 1.00 (reference) | 0.047 | 0.59 (0.19-1.84) | 0.396 |
| One site only | 0.49 (0.24-0.99) | |||
| Bone only site | ||||
| No | 1.00 (reference) | 0.076 | 0.79 (0.17-3.05) | 0.843 |
| Yes | 0.49 (0.22-1.09) | |||
| Liver involvement | ||||
| No | 1.00 (reference) | 0.010 | 2.32 (1.22-4.40) | 0.010 |
| Yes | 2.32 (1.22-4.40) | |||
| Age, years | 10.002 (0.98-1.03) | 0.902 | ||
| Menopausal status | ||||
| Postmenopausal | 1.00 (reference) | 0.899 | ||
| Premenopausal | 1.05 (0.55-2.00) | |||
| Disease status | ||||
| Recurrence | 1.00 (reference) | 0.666 | ||
| De novo | 1.14 (0.64-2.04) | |||
| Bone-only disease | ||||
| Yes | 1.00 (reference) | 0.080 | ||
| No | 0.49 (0.22-1.09) | |||
| Visceral involvement | ||||
| No | 1.00 (reference) | 0.098 | ||
| Yes | 1.73 (0.90-3.29) |
HR, hazard ratio; CI, confidence interval.
The multivariate analysis identified liver involvement as the only significant independent variable that predicted a more than two-fold increase in the risk of progression or death (HR, 2.32; 95% CI, 1.22-4.40; P=0.010).
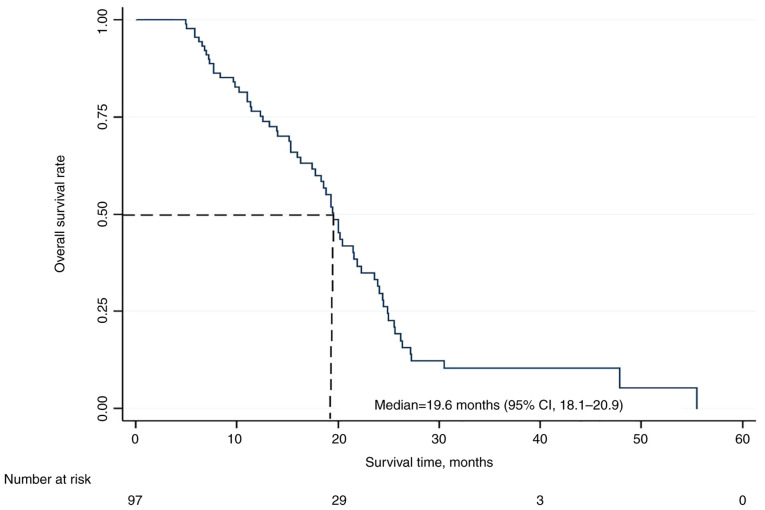
At the time of collecting data at the end of February 2021, 63 patients (65%) had died due to disease progression, with no mortality attributed to therapy. The remaining 34 patients (35%) were still alive with or without evidence of disease. The median OS time was 19.6 months (95% CI, 18.1-20.9), with 12- and 24-month OS rates of 75 and 30%, respectively (Fig. 2). At the time of writing this manuscript in July 2021, all the remaining patients had died.
Figure 2.
Kaplan-Meier survival curve for overall survival. CI, confidence interval.
Discussion
The treatment of HR+ advanced/metastatic breast cancer is challenged by the development of endocrine resistance over the disease course. The approval of the use of CDK4/6 inhibitors for this subtype represents a major therapeutic advance in this setting. Palbociclib combined with ET, aromatase inhibitors or fulvestrant is an important therapeutic option for patients with metastatic breast cancer (13,14,16).
The present study involved a population of patients with disease progression following prior ET for advanced disease. Data was extracted from the medical records of patients treated with palbociclib and ET. A total of 66% of patients had two or more metastatic sites, with liver involvement in 58% of patients. Patients in this series were heavily pretreated for metastatic disease (55% received systemic chemotherapy and 49% received two or more lines of prior ET).
All patients (100%) started treatment of palbociclib at 125 mg/day, and the majority (71.8%) remained on this dose. Overall, dose adjustments were required in only 30%. The dose adjustment was similar to that reported in the PALOMA-3 trial (31.6%), where patients with prior exposure to ET in the metastatic setting were treated with palbociclib and fulvestrant (14). In the current series, an ORR of 30% and a clinical benefit of 52% were demonstrated. These results compared favorably with the outcome reported in the PALOMA-3 study, where the rates were 10.4 and 34%, respectively (14). The reason for the poorer OS results despite the favorable PFS and ORR rates is not clear. However, the present patient population was heavily pretreated, 66% of patients had two or more metastatic sites, and 58 and 19% had liver and brain involvement, respectively. In the PALOMA-3 trial (14), patients with uncontrolled brain metastases were excluded from the study. Furthermore, for the present study, the initial tumor stage of the patients had no value in the metastatic setting, as all pivotal studies involving CDK4/6 inhibitors had also not included or reported on the initial tumor stage (13,14,16,17).
In the present study, the patients exhibited a median PFS time of 16.3 months (95% CI, 11.4-21.2), with 58% of patients remaining progression-free at 12 months. The current median PFS time also compares favorably with the median PFS time of 9.5 months reported in the final analysis of the PALOMA-3 study (17). The poor outcome associated with liver involvement (in 58% of patients) was also shown in a Japanese series that examined the efficacy of palbociclib in a similar setting (18). This observation is relevant considering the rare association between palbociclib use and fatal liver failure (19). However, while a palbociclib trial reported a few liver-related adverse events (20), palbociclib-related fatal liver toxicity has not been previously reported. Liver injury is not unique to palbociclib, as it has also been shown in association with other CDK4/6 inhibitors such as abemaciclib (21) and ribociclib (22).
The reported median OS time was 19.6 months (95% CI, 18.1-20.9) in the present study, which was much shorter than the 34.9 months recently reported in the PALOMA-3 trial (13). The reason for the poorer OS time despite the favorable PFS and ORR is not clear. However, again, the heavy pre-treatment, large number of metastatic sites, and high percentage of liver and brain involvement may have been a factor. In the PALOMA-3 studies, patients with uncontrolled brain metastases were excluded from the study.
Data reported from the current population demonstrate several strengths. First, a number of the included patients had multiple comorbidities, including diabetes mellitus, hypertension, and coronary vascular disease, representing a real-world patient population that may not necessarily be included in clinical trials. In a recently reported systematic review, He et al (23) showed that four out of five trials excluded at least half of the patients. In the breast cancer trials, 56% of patients were excluded for associated clinical comorbidity conditions. Second, to the best of our knowledge, the present series represents the only available experience in the region that examined the efficacy of palbociclib combined with ET for advanced breast cancer.
Several limitations exist in the current study, which may affect the results. First of all, due to the retrospective nature of the study, certain relevant information, such as patient performance status and tumor grade, was missing, and accurate data on performance status were not available. Furthermore, neither the exact frequency of adverse event rates nor the impact of the combined regimens on quality of life could be ascertained.
In conclusion, despite the retrospective nature of the current analysis and the inherent limitations, the presented data illustrate a favorable real-world experience of using palbociclib combined with appropriate ET in the management of metastatic HR+/HER2- breast cancer after failure of prior ET and systemic chemotherapy.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MHA and MMA conceptualized and designed the work and wrote the initial draft of the manuscript. AMAl, SNM, AMAb, MSA, MHA, AAR, AMB, AYS, MAK and EMI helped in the acquisition, analysis and interpretation of data for this case report. SSA did the literature survey, aligned the analysis in context to the current literature and drafted, edited, and revised the manuscript in its final form. MHA, MMA and SSA confirm the authenticity of all the raw data. All authors reviewed the final revised version of the manuscript and approved it for submission. All authors have agreed to be accountable for their contributions in this intellectual work. All authors have read and approved the manuscript.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of King Abdullah International Medical Research Center, a research wing of King Saud Bin Abdul Aziz University for Health Sciences (Jeddah, Saudi Arabia; approval no. SP20/068/J).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Althubiti MA, Nour Eldein MM. Trends in the incidence and mortality of cancer in Saudi Arabia. Saudi Med J. 2018;39:1259–1262. doi: 10.15537/smj.2018.12.23348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, Cronin KA. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(dju055) doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–1568. doi: 10.1001/jamaoncol.2018.2706. Global Burden of Disease Cancer Collaboration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, Dowsett M, et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13:417–430. doi: 10.1038/nrclinonc.2016.26. [DOI] [PubMed] [Google Scholar]
- 6.Sherr CJ, Roberts JM. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 8.Roberto M, Astone A, Botticelli A, Carbognin L, Cassano A, D'Auria G, Fabbri A, Fabi A, Gamucci T, Krasniqi E, et al. CDK4/6 inhibitor treatments in patients with hormone receptor positive, Her2 negative advanced breast cancer: Potential molecular mechanisms, clinical implications and future perspectives. Cancers (Basel) 2021;13(332) doi: 10.3390/cancers13020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rugo HS, Diéras V, Gelmon KA, Finn RS, Slamon DJ, Martin M, Neven P, Shparyk Y, Mori A, Lu DR, et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: Results from the PALOMA-2 trial. Ann Oncol. 2018;29:888–894. doi: 10.1093/annonc/mdy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma S, O'Shaughnessy J, Burris HA, Campone M, Alba E, Chandiwana D, Dalal AA, Sutradhar S, Monaco M, Janni W. Health-related quality of life of postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treated with ribociclib + letrozole: Results from MONALEESA-2. Breast Cancer Res Treat. 2018;170:535–545. doi: 10.1007/s10549-018-4769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth CM, Karim S, Mackillop WJ. Real-world data: Towards achieving the achievable in cancer care. Nat Rev Clin Oncol. 2019;16:312–325. doi: 10.1038/s41571-019-0167-7. [DOI] [PubMed] [Google Scholar]
- 12.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 13.Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 14.Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, Huang Bartlett C, Zhang K, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 17.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 18.Odan N, Kikawa Y, Matsumoto H, Minohata J, Suwa H, Hashimoto T, Okuno T, Miyashita M, Saito M, Yamagami K, Takao S. Real-world outcomes of treating advanced breast cancer patients with palbociclib: A multicenter retrospective cohort study in Japan-the KBCOG-14 study. Breast Cancer (Auckl) 2020;14(1178223420983843) doi: 10.1177/1178223420983843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vuppalanchi R, Saxena R, Storniolo AMV, Chalasani N. Pseudocirrhosis and liver failure in patients with metastatic breast cancer after treatment with palbociclib. Hepatology. 2017;65:1762–1764. doi: 10.1002/hep.28720. [DOI] [PubMed] [Google Scholar]
- 20. No authors listed: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2012. [PubMed] [Google Scholar]
- 21.Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, Park IH, Trédan O, Chen SC, Manso L, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 22.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martín M, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382:514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 23.He J, Morales DR, Guthrie B. Exclusion rates in randomized controlled trials of treatments for physical conditions: A systematic review. Trials. 2020;21(228) doi: 10.1186/s13063-020-4139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.