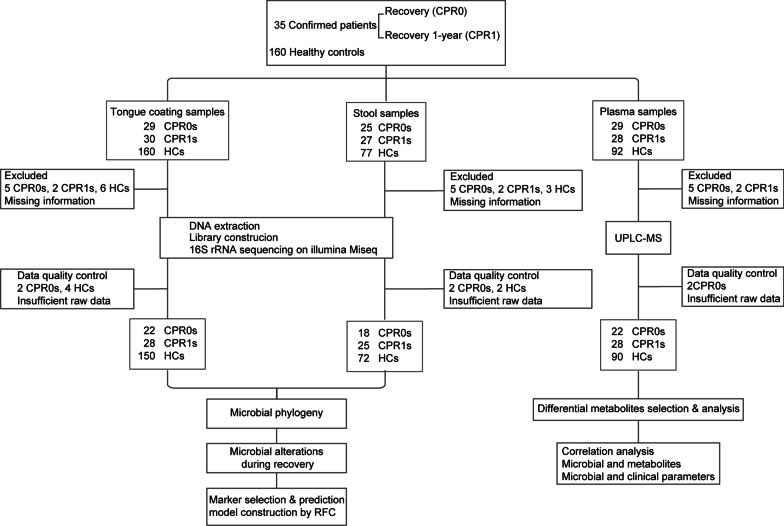
Fig. 1.
Study design and flow diagram. A total of 35 confirmed patients were recruited and completed a 1-year follow-up after recovery. In addition, 160 healthy controls (HCs) were enrolled. A total of 497 samples were prospectively collected, including 219 tongue-coating samples, 129 stool samples and 149 plasma samples. In addition, 455 samples were included for further analysis after strict inclusion and exclusion criteria. 16S rRNA MiSeq sequencing was conducted on tongue-coating samples and stool samples, and untargeted metabolomics testing on liquid chromatography-mass spectrometry (LC–MS) analysis was carried out on plasma samples. CPR0 confirmed patients recover at discharge, CPR1 confirmed patients recover 1 year, HCs healthy controls, UPLC-MS ultra-performance liquid chromatography-mass spectrometry, RFC random forest model