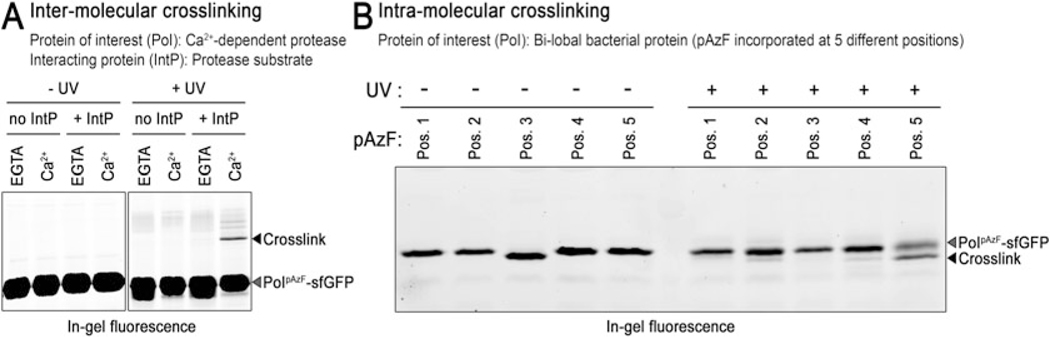
Fig. 3.
SDS-PAGE analysis of LapG photo-cross-linking. (a) Inter-peptide cross-linking: a fluorescently labeled, covalently cross-linked adduct with slower electrophoretic mobility is observed only when the LapG proteasesfGFP containing pAzF is illuminated with UV light in the presence of its substrate LapA (i.e., “IntP”) and calcium. This observation demonstrates calcium is required for protease–substrate interaction. (b) Intra-peptide cross-linking: pAzF was incorported at 5 different positions into a protein containing two structural lobes. Only when AzF was located as “position 5” (Pos. 5) was a new intra-peptide cross-linked species with faster electrophoretic mobility observed upon UV light illumination, indicating this residue (and not the others) is at the interface of two folded sub-domains