Highlights
-
•
Chronic migraine is a primary headache disorder associated with a substantially higher burden of disease, higher number of comorbidities, and higher social impact.
-
•
The factors which may predispose to the process of migraine progression include high frequency of migraine attacks, medication overuse, comorbid pain syndromes, and obesity.
-
•
The neuronal hyperexcitability in trigeminal nociceptive system is the plausible mechanism leading to the progression.
-
•
The possible mechanisms leading to this hyperexcitability include persistent sensitization caused by repetitive and prolonged trigeminal nociceptive activation and the decrease in endogenous brainstem inhibitory control.
-
•
The combination of increased pain matrix connectivity, including hypothalamic hyperactivity and a weak serotonergic system, may contribute to migraine chronification.
Keywords: Chronic migraine, Migraine progression, Trigeminal system, Neuronal hyperexcitability, Endogenous pain control system, Headache
Abstract
Chronic migraine is one of the most devastating headache disorders. The estimated prevalence is 1.4–2.2% in the population. The factors which may predispose to the process of migraine progression include high frequency of migraine attacks, medication overuse, comorbid pain syndromes, and obesity. Several studies showed that chronic migraine results in the substantial anatomical and physiological changes in the brain. Despite no clear explanation regarding the pathophysiologic process leading to the progression, certain features such as increased sensory sensitivity, cutaneous allodynia, impaired habituation, identify the neuronal hyperexcitability as the plausible mechanism. In this review, we describe two main mechanisms which can lead to this hyperexcitability. The first is persistent sensitization caused by repetitive and prolonged trigeminal nociceptive activation. This process results in changes in several brain networks related to both pain and non-pain behaviours. The second mechanism is the decrease in endogenous brainstem inhibitory control, hence increasing the excitability of neurons in the trigeminal noceptive system and cerebral cortex. The combination of increased pain matrix connectivity, including hypothalamic hyperactivity and a weak serotonergic system, may contribute to migraine chronification.
Introduction
Chronic migraine is one of the most devastating headache disorders. According to the International Classification of Headache Disorders (3rd edition) (ICHD-3), migraine is classified into two distinct categories depending on the clinical course, namely episodic migraine (EM) which is defined as having<15 headache days per month and chronic migraine (CM) characterised by those having 15 or more headache days per month for more than three months, with at least eight days having the characteristic of migraine features (IHS, 2018). Although both categories of migraine share many features including: 1) at least five recurrent migraine attacks without aura, 2) migraine duration of 4–72 h if left untreated, 3) at least two of the following pain characteristics: unilateral, pulsing, moderate or severe intensity, or exacerbated by routine physical activity, and 4) at least one of the following symptoms: nausea and/or vomiting or both photophobia and phonophobia. CM is distinguished from EM by its substantially higher burden of disease, higher number of comorbidities, especially psychiatric comorbidity, and higher overall cost and healthcare consumption (Adams et al., 2015, Negro et al., 2019).
The question remains as to whether chronic and episodic migraine are separate diseases or are a continuum of the same disease spectrum (Aurora, 2009, Aurora and Brin, 2017). Until now, there has been no evidence that supports the occurrence of CM without transformation from EM. Therefore, the main focus of the treatment strategy is to prevent progression. It is estimated that EM progresses to CM at a rate of 2.5% per year and may be underestimated due to the arbitrary 15-day period according to the migraine criteria (Bigal et al., 2008, Ishii et al., 2021). At the moment, migraines are diagnosed on clincal presentation; however, potential biomarkers are needed to help distinguish migraines earlier. A recent multicenter, longitudinal study done by Ishii et al. found that the use of 15 days/month does not capture the burden of illness and/or treatment options to differentiate EM from CM (Ishii et al., 2021). Understanding the neurobiological core of migraine progression can lead to potential biomarker discovery and contribute future migraine research. Here, we will be focusing on two main aspects: clinical progression of migraines and pathophysiology of migraine progression.
Clinical progression of migraines
Migraine clinical courses
Migraine as a linear progressive pattern
Various observational studies have identified and classified migraine patterns into 4 main courses: clinical remission, partial clinical remission, persistent and progressive (Bigal and Lipton, 2008a, Bigal and Lipton, 2008b).
Clinical remission is a clinical course in which a patient with migraine headache tends to remain symptom free for an extended period of time or even have no headache at all after a certain time point. Although no guideline has established the duration of the headache-free period, many clinical studies have used a 1-year symptom-free period as the duration of remission (Lipton et al., 2007, Silberstein et al., 2007). In the American Migraine Prevalence and Prevention (AMPP) study, the rate of remission is around 10% (Lipton et al.2007). Factors related to migraine remission have not been well studied, especially in EM; however, out of the existing remission studies, most have focused on CM, demonstrating that older age, male gender, fewer headache days and absence of allodynia are key factors for headache remission (Scher et al., 2003, Buse et al., 2010, Manack et al., 2011).
Partial clinical remission refers to the condition in which some features of migraine attacks, such as frequency or severity, are diminished. Partial remission patients will experience milder migraine features and symptoms during a 1-year period. Epidemiologic data shows the prevalence of this migraine category to be around 3% of all cases studied (Lipton et al.2007). Factors involved in partial remission are also related to age, demonstrating that advanced age is associated with fewer attacks and more atypical headache presentations (Bigal and Lipton, 2006).
Persistent migraine is defined as having constant but slightly fluctuating frequency, intensity and accompanying symptoms (nausea, vomiting, etc.) of migraine headache on a regular basis for years. In addition, persistent migraine does not show functional or anatomical changes (Bigal and Lipton, 2008a). It still remains unknown as to why this type of migraine does not remit or progress. Further studies are warranted to understand this phenomenon.
Progressive migraine is the most studied clinical course of migraine type due to its morbidity. Key features include progressive increases in the number and intensity of attacks, autonomic disturbance and allodynia which over time, leads to chronic migraine (Bigal and Lipton, 2008a, Manack et al., 2011, Katsarava et al., 2012, Torres-Ferrús et al., 2017). Apart from clinical changes, several studies have pointed out functional and anatomical changes within the brain. It is believed that alteration within the trigeminal nociceptive system, along with modulation of the brainstem, are key physiological changes within the brain circuitry. Furthermore, the occurrence of structural changes in progressive migraine makes for interesting markers in migraine chronification (Su and Yu, 2018, Eikermann-Haerter and Huang, 2021).
Migraine as a fluctuating pattern
The recent study by Serrano et al. questioned the 15 or more days per month for 3 months chronic migraine diagnostic criteria and found that the nature of migraine is more of a fluctuating course than constant. An estimated random-effect variance model found that there are variabilities in headache days within the EM and CM groups. In other words, when using this criterion, patients could experience CM in one month and EM in other months. Hence, the authors concluded that the nature of chronic migraine may not be clearly represented with the 15-day criteria due to the similar biology of the 2 specified groups (Serrano et al.2017).
Types of progression
Changes in migraine progression can be observed over time. The most dramatic changes can be seen clinically while, subtle changes occurring within brain processing or other physiological changes are more difficult to detect. To confirm these alterations, investigations including functional neuroimaging and the use of biomarkers, albeit still undetermined, are needed.
Clinical progression
Different clinical features in CM and EM are mainly investigated in epidemiologic studies. A summary of the main clinical outcomes is presented in Table 1. As mentioned above, clinical features are observable; however, some clinical features are subjective and might be over-reported in migraine patients.
Table 1.
Summary of key epidemiologic studies in the progression of migraine.
| Studies | Study Type | Study design | Results of factors associated with the transformation of migraine | Ref | |
|---|---|---|---|---|---|
| Caffeine intake | Population-based case control study | Episodic Headache (N = 507) | Chronic Daily Headache (N = 209) | High caffeine consumption OR = 1.50, p = 0.05 | Scher et al., 2004 |
| The American Migraine Prevalence and Prevention Study (AMPP) - Medication usage |
Longitudinal study with cross-sectional surveys | Episodic Migraine (N = 6805) | Transformed Migraine (N = 209) | barbiturates (OR = 2.06, (1.3–3.1) opiates OR = 1.98, (1.4–2.2) |
Bigal et al. 2008 |
| The International Burden of Migraine Study (IBMS) | Prospective multicenter cohort study | Episodic Migraine (N = 8227) | Chronic Migraine (N = 499) | Headache intensity, p < 0.001 Headache pain severity p < 0.001 moderate/severe pain, p < 0.001 MIDAS, p < 0.001 MSQ, p < 0.001 PHQ-4, p < 0.001 |
Blumenfeld et al. 2011 |
| The American Migraine Prevalence and Prevention Study (AMPP) | Longitudinal study with cross-sectional surveys | Episodic Migraine (N = 11,249) | Chronic Migraine (N = 655) | Obesity OR = 1.24 (1.03 to 1.50) Anxiety OR = 1.80 (1.51 to 2.15) PHQ-9 Depression OR = 2.00 (1.67 to 2.40) High cholesterol OR = 1.46 (1.23 to 1.73) Heart disease or angina OR = 1.43 (1.08 to 1.90) |
Buse et al. 2010, |
| Comorbid pain syndrome | Cross-sectional study | Severe headache (N = 29,721) |
Non-severe headache (N = 160,255) |
Temporomandibular Joint and Muscle Disorder OR = 7.0 (6.6–7.5) Neck Pain OR = 5.0 (4.8–52.) Low back pain OR = 3.6 (3.5–3.7) Joint Pain OR = 2.3 (2.2–2.4) |
Plesh et al. 2012 |
| The American Migraine Prevalence and Prevention Study (AMPP) - Headache factors and depression |
Longitudinal study with cross-sectional surveys | Episodic Migraine N = 6,657, year 2005 N = 6,852, year 2006 |
Chronic Migraine N = 160, year 2005 N = 144, year 2006 |
Headache Frequency OR = 1.29 (1.21–1.36) PHQ-9 OR = 1.65 (1.12–2.45) |
Ashina et al.2012 |
| The American Migraine Prevalence and Prevention Study (AMPP) - Headache factors |
Longitudinal study with cross-sectional surveys | Episodic Migraine (N = 10,763) | Chronic Migraine (N = 795) | Headache days OR = 7.31 (6.98, 7.66, P < 0.0001) MIDAS score OR = 5.36 (4.88, 5.90, P < 0.0001) Allodynia score OR = 1.21 (1.11, 1.31, P < 0.0001) PHQ-9 score OR = 1.52 (1.42, 1.63, P < 0.0001) |
Lipton et al. 2014 |
| The American Migraine Prevalence and Prevention Study (AMPP) - Nausea |
Longitudinal study with cross-sectional surveys | Persistent frequent headache-related nausea group (N = 1389) |
No or low frequency nausea group (N = 877) |
risk of progression to CM OR = 2.24, (1.07–4.70) P = 0.033 | Reed et al., 2015 |
| The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study | Longitudinal study with cross-sectional surveys | Episodic Migraine (N = 15,313) | Chronic Migraine (N = 1476) | Female OR = 1.52 (1.33–1.75), p < 0.001 Obesity OR = 1.34 (1.21–1.50), p < 0.001 MIDAS score RR = 4.63 (4.31–4.98), p < 0.001 Headache frequency RR = 6.49 (6.21–6.78), p < 0.001 PHQ-9 Depression OR = 3.05 (2.74–3.40), p < 0.001 Generalized anxiety disorder − 2.40 (2.16–2.67), p < 0.001 |
Adams et al., 2015 |
| Chronic migraine and Obesity: Systematic review and meta-analysis of observational studies | Systematic review and meta-analysis | Outcome: Chronic migraine Exposure: Pre-obesity/Obesity |
Pre-obesity 1.39; 95% CI, 1.13–1.71; P = 0.002 Obesity 1.75; 95% CI, 1.33–2.29; P < 0.001 |
Ornello et al, 2015 | |
| Hospital Universitari Vall d’Hebron (HUVH), Spain | Prospective cohort study | Episodic Migraine (N = 855) | Chronic Migraine (N = 254) | Insomnia 39.6% vs 56.7%, p < 0.001 Anxiety disorder 55.8% vs 68.5%, p < 0.001 Depression 7% vs 12.2% 0.008, p < 0.001 |
Torres-Ferrús et al., 2017 |
| The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study - Sleep disorder |
Longitudinal study with cross-sectional surveys | Episodic Headache (N = 11,659) |
Chronic Migraine (N = 1111) | High risk for sleep apnea 51.8% vs 35.6%; P < 0.001 Sleep disturbance 26.9% vs 24.3%; P < 0.001 Snoring 33.9% vs 32.1%; P < 0.001 Shortness of breath 29.8% vs 20.6%; P < 0.001 Somnolence 23.4% vs 21.2%; P < 0.001 Sleep adequacy 24.2% vs 22.1%; P < 0.001 |
Buse et al, 2019 |
| Factors associated to chronic migraine with medication overuse: A cross-sectional study |
Cross-sectional study | Episodic Headache (N = 156) |
Chronic Migraine (N = 162) | Physical activity (OR 0.42, 95% CI 0.19–0.91, p = 0.029) Age at onset of migraine (OR 0.94, 95% CI 0.89–0.98, p = 0.016) At least one migraine preventive medication (OR 2.36, 95% CI 1.18–4.71, p = 0.014) Depression (OR 2.91, 95% CI 1.25–6.73, p = 0.012) Insomnia associated with the use of hypnotics (OR 5.59, 95% CI 1.65–18.93, p = 0.006) Traumatic head injuries (OR 3.54, 95% CI 1.57–7.99, p = 0.002) Snoring (OR 2.24, 95% CI 1.05–4.79, p = 0.036) Combined oral contraceptives (OR 3.38, 95% CI 1.10–10.3, p = 0.031 |
Viana et al. 2018 |
| Association between periodontitis and chronic migraine: a case-control study |
Case-control study | Episodic Migraine (N = 91) |
Chronic Migraine (N = 102) | Chronic periodontitis OR = 2.4; 95% CI 1.2–4.7; p = 0.012 | Ameijeira et al. 2019 |
| The migraine in America symptoms and treatment (MAST) study |
Prospective cohort study | Prospective study of 15,133 people with migraine and and 77,453 controls. Assess increased migraine headache days and associated comorbidities |
Significant outcome (P < 0.001) includes gastric ulcers/GI bleeding diabetes anxiety depression insomnia asthma and allergies/hay fever |
Buse et al. 2020 | |
| Predictors of episodic migraine transformation to chronic migraine: A systematic review and meta-analysis of observational cohort studies | Systematic review and meta-analysis | Predictor of chronic migraine using the fixed effect model | Depression RR = 1.58 [1.35, 1.85] Monthly headache day frequency greater than 5 days/month RR = 3.18 [2.65, 3.82] Monthly headache day frequency greater than 10 days per month RR = 5.95 [4.75, 7.46] Allodynia RR = 1.40 [1.23, 1.59] |
Xu et al. 2020 | |
| The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study - Psychiatric comorbidities |
Longitudinal study with cross-sectional surveys | Episodic Headache (N = 15,312) |
Chronic Migraine (N = 1476) | Depression 56.6% vs 30.0%; P < 0.001 Anxiety 48.4% vs 28.1%; P < 0.001 Coexisting depression and anxiety 42.0% vs 20.8%; P < 0.001 |
Lipton et al. 2020 |
| Searching for Predictors of Migraine Chronification: a Pilot Study of 1911A > G Polymorphism of TRPV1 Gene in Episodic Versus Chronic Migraine |
Cross-sectional study | Episodic Migraine (N = 27) |
Chronic Migraine (N = 19) |
TRPV1 SNP gene Episodic migraine genotype (AA 33%, AG 56%, GG 11% and AA 34%, AG 46%, GG 20%) Vs Chronic migraine genotype (AA 68%, AG 32%, GG 0%) P < 0.05 |
Yakubova et al. 2021 |
Clinical features
Headache frequency
Observational studies included in this review have found that one of the most significant factors leading to CM is the increase in headache frequency, measured by the number of headache days per month. Two longitudinal analyses of the American Migraine Prevalence and Prevention (AMPP) studies, done on separate occasions, have also demonstrated these findings. Lipton et al. compared EM and CM patients during 2005–2009 and reported that the number of headache days for CM versus EM had an odds ratio of 7.31 (6.98, 7.66, p < 0.0001) (Lipton et al.2014). In the other AMPP study, Ashina et al. reported an odds ratio of 1.29 (1.21–1.36, p 0.0001) (Ashina et al.2012). The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study also showed a significant difference in headache frequency days with an odds ratio of 6.49 (6.21–6.78, p 0.001) (Adams et al.2015). Therefore, there is an association between headache frequency and chronification of migraine. A recent systematic review and meta-analysis found that a monthly headache day frequency of more than 10 days had a risk ratio of 5.95. While a monthly headache day frequency of more than 5 days had a risk ratio of 3.18 in predicting the transformation of CM (Xu et al.2020). It has also been suggested that migraine preventive medication should be started if patients have more than 2 headache days per week to prevent CM and medication overuse headaches (MOH) (Sun-Edelstein et al.2021).
Cutaneous allodynia
Cutaneous allodynia is defined as an abnormal perception of pain in response to non-noxious stimuli. This clinical feature has been reported to occur in 63.2% of migraine patients (Lipton et al. 2008). Patients with CM had higher allodynia scores than those with EM (OR = 1.21, 95% CI 1.11–1.31, P.0001) (Lipton et al.2014). In the Leiden University Migraine Neuro-Analysis (LUMINA) prospective, a 6-month longitudinal study showed that allodynia was an independent predictor for an increased number of migraine days (Louter et al. 2013). In a systematic review and meta-analysis using the fixed effect model to predict the transformation of EM to CM, the authors reported only a slight increase in risk (RR = 1.40, 95% CI 1.23–1.59), citing the study’s inclusion criteria for their marginal results. (Xu et al.2020). Despite this, cutaneous allodynia still remains a key feature in the progression of migraine.
Nausea
Migraine diagnosis often includes other symptoms such as nausea, which was once believed to vary with the frequency of headaches. However, findings suggest that headache frequency does not always correlate with nausea (Lipton et al., 2013). In a prospective cohort study, migraine patients with persistent frequent nausea were more prone to develop chronic migraine (OR 2.2, 95% CI 1.7–4.7). After adjustment of the confounder, nausea was found to be affiliated with chronic migraine progression and may also be part of the causal pathway of chronification. (Reed et al., 2015).
Photophobia
Also called photic allodynia, photophobia is a common characteristic of trigeminal hypersensitivity. In a longitudinal cohort study, photophobia was associated with a poor outcome of migraine (OR = 3.93, 95%CI 0.38–40.48); however, the results did not reach statistical significance (Ashina et al. 2010). A cross-sectional study conducted in women with chronic migraine and without migraine found that those with CM have higher levels of photophobia and phonophobia than non-migraine (p < 0.05) (Pinheiro et al.2021).
Migraine-like headaches or tension-type headaches
According to the ICHD-3, mandatory diagnosis of chronic migraine requires at least 8 migraine days per month while, the remaining days may be other or less severe types of headache such as tension-type or milder migraine-like headaches; however, the debate has been made as to whether these other headaches are actually mild migraine attacks or true tension-type headaches (Chalmer et al.2020). To date, no solid evidence has yet confirmed the type of headache but inclusion of these other headache types in the study acknowledges lower severity of CM on certain days (May and Schulte, 2016), which may present easier treatment opportunities.
Physiological progression represented by functional neuroimaging
Functional neuroimaging plays a crucial role in the study of migraine since migraine itself is a physiological dysregulation. An early study in 1995 measured cerebral blood flow during and after migraine attacks in nine patients. The group was the first to report activation in the brainstem and cerebral cortex. Brainstem hyperperfusion persisted despite sumatriptan injection (Weiller et al., 1995). In another study, eight refractory CM patients who were implanted with occipital nerve stimulator underwent PET scan. During headache periods, increased regional blood flow was seen in the dorsal pons and the anterior cingulate cortex (Matharu et al., 2004). These areas were identified as either the pain processing pathway or the pain modulating system. More recent findings have found distinct regions involving in CM, including the basal ganglia and the limbic system (Maniyar and Goadsby, 2013). In a PET study in CM, it was found that interictal glucose metabolism increased in the pons and right temporal cortex but decreased bilaterally in the medial frontal, parietal, somatosensory cortices, and the caudate nuclei. Authors concluded that the brainstem inhibitory process is diminished in CM (Aurora et al.2007).
The hypothalamus is involved in the pathogenesis of premonitory symptoms of migraine and may serve as a migraine generator. Changes in hypothalamic activity have also been found in patients with CM. Compared with normal controls, patients with CM, but not EM, showed a significant increase in activation of the anterior hypothalamus (Schulte et al. 2017). The same area showed more activity in CM patients with headaches at time scanning compared to patients with EM with headaches during scanning. Lerebours et al demonstrated a significant connectivity between anterior hypothalamus and spinal trigeminal nucleus in patients with CM but these two regions were not connected in the episodic group. Since the hypothalamus plays a role in the premonitory phase, the authors suggest that the increased activation of the anterior hypothalamus locks CM in the preictal phase (Lerebours et al.2019).
In addition to its specific location within the pain pathway, connectivity between these regions have also been mentioned in current studies; two of which have shown that the periaqueductal grey (PAG) is interconnected with multiple areas of the brain. As migraine frequency increases, certain areas increased their signal strength, including the anterior insular cortex, the supramarginal gyrus and the hypothalamus, while some connectivity was decreased in the prefrontal cortex, anterior cingulate gyrus and the amygdala (Kong et al., 2010, Mainero et al., 2011).
Anatomical progression
Although migraine is recognized as a disorder of physiologic dysregulation, anatomical changes in the nervous system have been clearly demonstrated. Significant anatomical changes in migraine include changes in brain volume, metal deposition, and white matter changes.
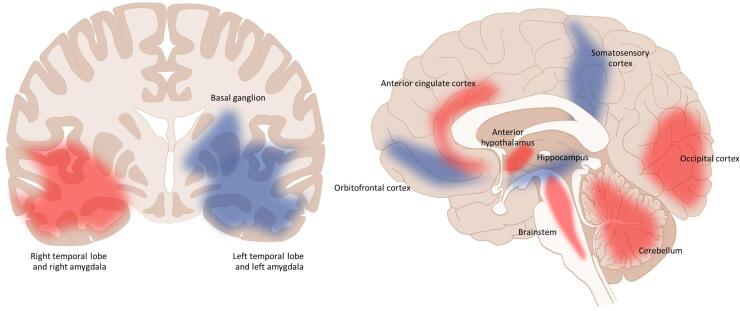
Morphological changes within the brain are studied using voxel-based morphometry (VBM) as a quantitative measurement technique that detects the concentration of brain tissue within the specific loci in comparison with control brain specimens (Ashburner and Friston, 2000). It enables the quantitative estimation of volume loss or thickening or gain in specific areas of the brain. This method is not new and has been established in other types of medical research including migraine (Valfrè et al., 2008). In CM, VBM findings suggest that volume changes occur within the pain pathway and cognitive pathway. In this regard, these changes may help explain ongoing pain and clinical symptoms in patients. Current studies comparing CM with EM or control were summarized (Fig. 1). Increased volumetric areas of the brain include the basal ganglion, right hippocampus, right amygdala, and the orbitofrontal cortex while, decreased volume of the brain includes the brainstem, cerebellum, left amygdala, anterior hypothalamus, occipital cortex, and anterior cingulate cortex. Controversial brain areas were the somatosensory area and temporal lobes (Valfrè et al., 2008, Bilgiç et al., 2016, Lai et al., 2016, Neeb et al., 2017, Coppola et al., 2017, Niddam et al., 2018, Chen et al., 2019). As some results were of controversy, an argument was made about the timing of the studies. In a longitudinal study, increased volume was reported in the early stages of CM; however, two years later, the volume was reported to decrease as the disease becomes more chronic (Liu et al.2017). In addition, most of the findings from our comparison showed a significant correlation between attack frequency and volume alteration (Valfrè et al., 2008, Coppola et al., 2017, Liu et al., 2017). It is believed that the alteration of brain volume is associated with the frequency of migraine attacks and duration of the disease.
Fig. 1.
Summary of voxel-based morphology studies, areas that increased in volume (blue) includes the basal ganglion (Neeb et al.), orbitofrontal cortex (Valfre’ et al., Lai et al.) and right hippocampus (Neeb et al.). Areas that decreased in volume (red) includes anterior cingulate cortex (Niddam et al., Valfre’ et al.), occipital cortex (Coppola et al., Lai et al.), Cerebellum (Bilgic et al., Lai et al.), anterior hypothalamus (Chen et al.), and the brainstem (Bilgic et al.). Controversial areas (blue and red) include the temporal lobe (Neeb et al.), amygdala (Neeb et al., Coppola et al., Valfre et al) and somatosensory cortex (Valfre et al., Neeb et al.). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Apart from the changes found in VBM, the accumulation of iron deposition in the PAG has been found in CM. PAG is involved in the descending brainstem modulating systems; therefore, dysfunction of this area could lead to the progression of migraine. In early studies, iron deposition was found in both EM and CM but was not found in controls. However, the difference in deposition between EM and CM was not different (Welch et al. 2001). In a recent study, CM showed greater iron deposition within the PAG compared with EM and control groups (Domínguez et al., 2019). Therefore, iron deposition could possibly be a marker of chronicity. It is suspected that iron deposition is a consequence of repeated migraine attacks, causing free radical oxidative stress and blood brain barrier leakage (Domínguez et al., 2019). Other areas are also affected by the same process, including the red nucleus, putamen, and the globus pallidus (Aurora and Brin, 2017).
Another interesting finding in CM is the presence of white matter lesions (WML). Well-known as a consequence of vascular precipitating risks and age, WML have predominantly been found up to 2–4 fold in migraine compared with normal controls (Swartz and Kern, 2004). In the Cerebral Abnormalities in Migraine, an Epidemiological Risk Analysis (CAMERA) population-based study, Kruit er al. found that the frequency of migraine was associated with WML progression (odds ratio, 2.6; 95% CI, 1.2–5.7) (Kruit et al., 2004). The study also adjusted cardiovascular risks to control the confounding factors. In a follow-up study, higher frequency and longer duration of migraine showed a higher risk of WML (Kruit et al., 2010). On the contrary, in the CAMERA-2 study, WML was not associated with the frequency of migraine (Palm-Meinders et al., 2012). It was argued that the CAMERA-2 study might have had lower attack frequency and fewer cases. Another study found that WML was associated with nausea and pain intensity during attacks (Negm et al., 2018). The exact pathophysiology has not been well established but is thought to be linked to the ischemic process and neuroinflammation. The role of ischemia is believed to be due to a decrease in cerebral blood perfusion during migraine attacks and therefore, can cause focal white matter ischemia (Olesen et al., 1990). It has also been studied that during migraine attacks, patients’ blood vessels are more prone to vasoconstriction. To date, studies in neuroinflammation in CM have not been well established. Of the existing reports, however, most have been based on findings in multiple sclerosis where cytokines and neuropeptides were found to have played a role in glial dysfunction and neurodegeneration (Datta et al., 2017).
Other associated factors
Depression
Depression is a common comorbidity for migraine sufferers. The occurrence of depression could alter a patient’s clinical course and prognosis. Therefore, early detection and optimal treatment strategy are crucial to achieving better quality of life. Epidemiology studies have pointed out the association between depression and CM (Buse et al., 2020). In a population-based retrospective matched cohort study done in Taiwan, it was found that the relative risk of depression was 1.88 (p < 0.0001) in CM than other migraines (Chen et al., 2012). After adjusting for sociodemographic variables and headache characteristics, data from the AMPP study found that depression was a significant predictor of CM (OR = 1.65, 95% CI 1.12–2.45) (Ashina et al., 2012). In addition, migraine sufferers with allodynia (as a marker of CM) were found to have a higher association with depression (Kao et al., 2014). Interestingly, depression also has bidirectional effects on migraine. Using two-way hazard ratio analysis, Breslau et al showed that a sex-adjusted hazard ratio of the first onset of major depression in persons with previous migraine was 2.35 (95% CI 1.84–3.01), while the hazard ratio for the first occurrence of migraine in persons with prior major depression was 2.75 (95% CI 2.17–3.48) (Breslau et al., 2000).
Previous studies have shown the relationship between migraine and depression and how they share similar mechanisms. For example, via the aminergic systems, which includes serotonin and dopamine. A study in a rat model using inflammatory soup (IS) to induce CM found that the rats demonstrated depression-like behavior. In addition, serotonin and dopamine in the prefrontal cortex of the IS rat were significantly lower compared with controls (Zhang et al., 2017). Real-world clinical settings have shown that selective serotonin reuptake inhibitors could treat both depression and CM. Another proposed mechanism is via psychiatric model called the “learned helplessness” model. This model was initiated to explain depression as a consequence of an unpredictable and uncontrollable event (Sheftell and Atlas, 2002). In this case, the frequency of migraine attacks represented the helplessness event which required attention in order to stop the ongoing process.
Chronic pain syndromes
Chronic migraine has been reported to coexist with a variety of chronic pain syndromes. A US National Interview Survey conducted between 2000 and 2005 discovered that among patients with severe headache or migraine, temporomandibular joint and muscle disorder, and neck pain had an OR of 7.0 (95% CI 6.6–7.5) and OR of 5.0 (95% CI 4.8–52), respectively (Plesh et al., 2012). The underlying mechanism is believed to be due to the activation of the trigeminovascular pathway. Furthermore, central sensitization may play a role in pain pathogenesis (Calhoun et al., 2010, Anderson et al., 2011). As a comorbidity, it is believed that chronic pain syndrome occurs due to shared physiology with migraine. Currently, there is still insufficient data and evidence to define a causal relationship.
Obesity
Multiple epidemiologic studies have demonstrated a correlation between obesity and chronic migraine. In an AMPP study, it was found that obesity was a factor associated with migraine chronification (OR = 1.24, 95%CI 1.03 to 1.50) (Buse et al., 2010). The CaMEO study showed similar results (OR = 1.34, 95%CI 1.21–1.50) (Adams et al., 2015). Later, a systematic review and meta-analysis of observational studies focusing on obesity found an association between obesity and CM. There is almost a 2-fold increase risk in developing chronic migraine for obese patients (RR = 1.75; 95% CI, 1.33–2.29; P 0.001), while there is a 1.39 times association in pre-obesity conditions (RR = 1.39; 95% CI, 1.13–1.71; P = 0.002) (Ornello et al., 2015). Despite these findings, the underlying pathophysiology has not been well established. Two of the most studied mediators are leptin and adiponectin, both adipokines, (a group of peptides involved in weight modulation, immunity, and insulin resistance) that are found mainly in fat tissue (Peterlin et al., 2016). In a cross-sectional study, serum levels of leptin and adiponectin significantly increased compared to EM in CM (Domínguez et al., 2018). Therefore, it is believed that adipokines are responsible for the inflammatory process leading to chronification.
Pathophysiology of migraine progression
Pathogenesis of migraine
To understand the possible pathogenesis of migraine progression, it is necessary to understand the key elements of the mechanism underlying migraine (for review please see Burstein et al., 2015, Dodick 2018). Migraine attack consists of multiple phases including premonitory, aura, headache and postdrome. Increased susceptibility in development of these phases can lead to progression or deterioration. Each phase has specific physiological mechanism which involves specific brain area. The symptoms during the premonitory phase of migraine, such as changes in appetite, altered sleep-wake rhythms, mood changes or changes in liquid tolerance and others, indicate transient dysfunction of the hypothalamus. Since the hypothalamus has wide connections to cortical and subcortical structures as well as brainstem nuclei that modulate nociceptive signaling, alteration of the hypothalamus can increase the susceptibility of cortical spreading depression (CSD) development as well as trigeminal nociception. CSD is thought to be a transient slowly propagated wave of depolarization followed by suppression of brain activity. This change in cortical activity is a mechanism underlying the migraine aura.
The headache phase involves activation of the trigeminovascular system. During the attacks of migraine, the trigeminal nociceptive system is intermittently sensitized. Increased sensitivity of trigeminal nociceptors that innervate the dural meninges, so called peripheral sensitization, is responsible for the exacerbation of intracranial headache pain due to physical activity and movement, as well as the pulsating nature of migraine head pain. Prolonged activity in trigeminal afferents will induce the state of increased sensitivity of central trigeminal neurons, resulting in central sensitization. This process accounts for scalp and forehead allodynia observed during a migraine attack. Sensitization of trigeminothalamic neurons is likely to account for the widespread cutaneous allodynia involving extracephalic regions.
The mechanism underlying fluctuation of the trigeminal nociceptive threshold in migraine patients is still unclear. The trigeminal nociceptive system is under the influence of brainstem modulation where several brainstem nuclei have axons that either increase or decrease the sensitivity of central trigeminal nociceptive pathways. An increase in regional cerebral blood flow in the mesencephalon and pons has been demonstrated during a migraine attack (Weiller et al., 1995). These changes persist even after headache termination, indicating their roles in either migraine generation or sustentation. The alteration of these brainstem nuclei may play a role in this process. The observation of the accumulation of iron in the PAG over the duration of illness reflects the relationship between these brainstem areas and the chronicity of migraine (Welch et al., 2001). Therefore, alteration of these brainstem nuclei may play a role in this process. In addition to nociception, the brainstem modulating system also influences cortical excitability and can modify the threshold of elicitation of CSD.
Pathogenesis of migraine progression
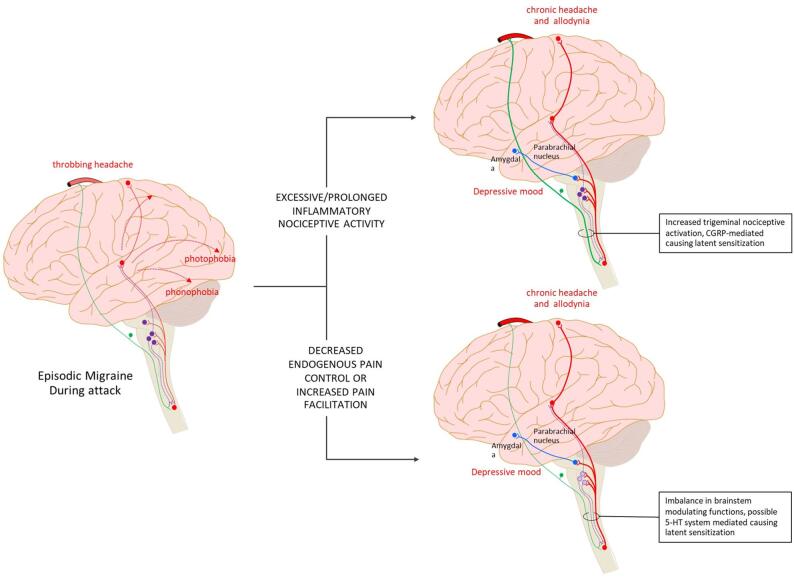
Both clinical features such as increased sensory sensitivity, cutaneous (cranial and extracranial) allodynia, etc. and neurophysiological findings such as impaired habituation, identify neuronal hyperexcitability as the pathophysiologic mechanism underling migraine progression. There are two main plausible mechanisms which can lead to this hyperexcitability. The first is an increase in the intrinsic excitability of the neurons in brain areas responsible for migraine pathogenesis, the process known as sensitization. This intrinsic neuronal change may be the result of repeated nociceptive activation. The second mechanism is the decrease in endogenous brainstem inhibitory control, hence increasing the excitability of neurons in the trigeminal nociceptive system and cerebral cortex (Fig. 2).
Fig. 2.
Possible mechansims leading to migaine progression. Hyperexcitabilty of neurons in trigeminal nociceptive pathway and other brian areas related to migraine patogeneisis plays important roles in migraine progression. Two main plausible mechanisms which can lead to this hyperexcitability are sensitization caused by repetitive and prolonged nociceptive activation, and the alteration in inhibitory control.
Repetitive nociceptive activation and sensitization
Increased sensitivity of neurons in the trigeminal nociceptive system is important in the pathogenesis of headache in migraine patients. The current concept is that neuropeptides released during migraine attack sensitize neurons with cell bodies in trigeminal ganglia. Calcitonin gene-related peptide (CGRP), the neurotransmitter released from nociceptive terminals, plays a crucial role in this process. An increased level of CGRP was demonstrated in the external jugular but not the cubital fossa blood of patients during migraine attacks which indicates that CGRP is regionally released in the cranial circulation (Goadsby et al., 1990). This ictal released CGRP can facilitate trigeminal nociceptive transmission and contributes to the development and maintenance of a sensitized, hyperresponsive state of the trigeminal ganglionic neurons. This peripheral sensitization has been accepted as a possible mechanism responsible for the pulsating nature of migraine head pain and the aggravation of the headache by routine physical activity.
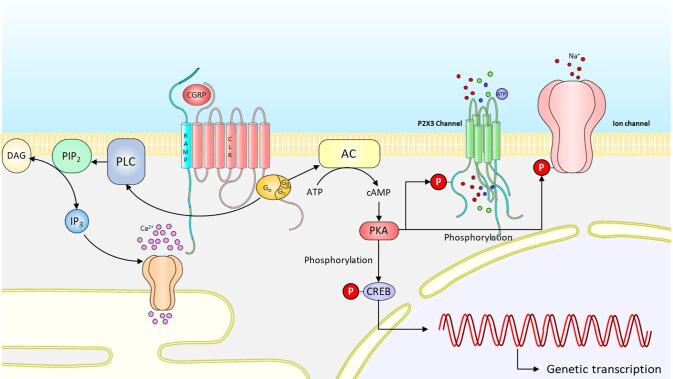
Binding of CGRP with its receptor complex activates multiple signaling cascades in the postsynaptic cells, including the activation of adenylate cyclase, with a subsequent increase in cAMP and activation of protein kinase A (PKA). This process results in the phosphorylation of multiple neuronal proteins. Phosphorylation of ion channels such as the P2X3 channel, along with the TRP receptor altering their conductance can lead to sensitization. Other targets include transcription factors, such as cAMP response element-binding protein (CREB) which can result in long-term change in neuronal function (Fig. 3). Interestingly, although CGRP plays an important role in nociceptive transduction, its receptors are not expressed on small nociceptive CGRP-releasing neurons. Instead, medium-sized trigeminal ganglion neurons (Aδ fibers) and satellite glial cells, express CGRP receptor components, calcitonin receptor-like receptor (CLR) and the receptor activity-modifying protein 1 (RAMP1) (Lennerz et al. 2008). The pattern of CGRP and CGRP receptor expression separates the ganglion neurons into CGRP-secreting and CGRP-responding fractions. This anatomical localization implies that CGRP might evoke activation of Aδ fibers or satellite glial cells, resulting in the increased expression of cytokines via activation of protein kinases. This potential crosstalk of signals between CGRP-secreting and a CGRP-responding cells involves intracellular mechanisms, including gene expression, leading to an increase in expression of inflammatory cytokines in dura mater, neuronal cell bodies and glial cells in the trigeminal ganglion (Messlinger et al 2020). Enhanced cytokine production and release, in or near neuronal cell bodies in the trigeminal ganglion, could act as a neuronal signal enhancer, causing neurogenic neuroinflammation and sensitization (Edvinsson et al 2019). As mentioned above, high frequency of migraine attacks is a risk factor of progression. An increase in migraine frequency can result in repeated release of CGRP, causing frequent neurogenic inflammation, prolonged activation and sensitization of trigeminal nociceptive terminals. -.
Fig. 3.
CGRP transduction cascade. Binding of CGRP with its receptor complex activates multiple signaling cascades in the postsynaptic cells, including the activation of adenylate cyclase, with a subsequent increase in cAMP and activation of protein kinase A (PKA). This process results in the phosphorylation of multiple neuronal proteins. Phosphorylation of ion channels such as P2X3 channel, TRP receptor alters their conductance and can lead to sensitization. Sustained CGRP release from central axons of trigeminal ganglionic neurons can trigger the activation of the mitogen-activated protein kinase (MAPK) signaling cascade. The activated kinase promotes the phosphorylation of the NR1 subunit of the NMDA receptor, causing changes in the channel transduction and excitability of postsynaptic neurons. Other targets include transcription factors, such as cAMP response element-binding protein (CREB) which can result in long-term change in neuronal function.
The role of CGRP in the process of migraine progression is supported by the clinical observations of high CGRP in patients with CM. Female patients with CM had higher blood CGRP levels compared with control healthy women, women with EM, and patients with episodic cluster headache. CGRP levels were more pronounced in those with a history of migraine with aura, compared to those only experiencing migraine without aura (Cernuda-Morollón et al., 2013). Interestingly, a follow-up study by the same investigators showed that the probability of being a responder to onabotulinumtoxin type A was 28 times higher in CM patients whose CGRP levels were above the threshold of 72 pg/mL (Cernuda-Morollón et al., 2014). In addition to plasma, high levels of CGRP have also been demonstrated in saliva and CSF (Jang et al., 2011, Van Dongen et al., 2017). It should be noted, however, that there was no difference in interictal CGRP level between CM, EM, and controls, as reported by Lee et al. (Lee et al., 2019). The role of CGRP in the pathogenesis of CM is further supported by the beneficial therapeutic effect of monoclonal antibody against CGRP or its receptor (Han et al., 2019). Reduction in the number of headache days in CM patients treated with monoclonal antibodies to CGRP (galcanezumab, fremanezumab, and eptinezumab) or the monoclonal antibody to the CGRP receptor (erenumab) was observed (Lipton et al., 2020, Lipton et al., 2021, Diener et al., 2021, Dodick et al., 2021).
In addition to CGRP, other neuropeptides which possibly play a role in the process of migraine progression include vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP). An animal CM model using repeated injection of nitroglycerin showed a significant increase in CGRP, VIP, PACAP and secretogranin (Anapindi et al., 2019). Clinical data also supported the involvement of VIP and PACAP in the CM pathogenesis. CGRP and VIP levels were significantly increased in CM population vs controls. Similar to CGRP, VIP levels were significantly increased in those who responded to onabotulinumtoxin type A, compared to the nonresponders (Cernuda-Morollón et al., 2014). A case-control study using multinomial modeling showed that VIP and PACAP increased the risk for CM, but not for EM, while CGRP did not predict CM or EM (Pérez-Pereda et al., 2020).
Lack of habituation and central sensitization
Lack of habituation
One proposed mechanism that leads to migraine progression is the inability to accommodate to stress placed on the organism. Kandel and Tauc found in their research on the physiological basis of memory storage in neurons, their work in habituation and its principles are defined as the decremental response to repeated stimuli (Kandel and Tauc.1965). The basis of using habituation in the study is due to the fact that the migraine habituation process is lacking when responding to repetitive non-noxious stimuli (Coppola et al.2013a). In proving that the migraine habituation process exists, multimodal physiologic studies have been used including evoked potentials, transcranial magnetic stimulation, and magnetoencephalography. A pioneer study in this field found that contingent negative variation (CNV) amplitudes (a potential that can be recorded on the brain cortical surface between two defined and contingent external stimuli) were higher in migraine sufferers compared to normal control during interictal migraine attacks (Kropp and Gerber, 1995). These findings suggest that the higher amplitude represents a lack of habituation. Repetitive transcranial magnetic stimulation studies have also shown similar results (Coppola, 2015).
In contrast to EM, CM shows higher cortical response in the form of reduced amplitude in laser-thermal evoked potential and is interpreted as a lower pain threshold than normal controls (de Tommaso et al. 2003). Another method used in CM is the blink reflex (BR). BR obtains information from peripheral and central components of the nervous system. It is a representative of the trigeminal nucleus caudalis activation pathway and lack of habituation (Unal et al.2016). In a comparative study using nociceptive BR, CM showed a more prolonged latency and smaller amplitudes, suggesting that this might be due to cortical hyperexcitability or lack of habituation (Sohn et al.2016). Another study using the same method found that migraine patients during migraine-free periods showed deficient BR habituation and an inverse relation to attack frequency (Di Clemente et al.2007). This was not thought to be due to sensitization. Interestingly, neurophysiological patterns of CM have shown some similarity to the ictal phase of EM. A somatosensory evoked potential study measuring high-frequency somatosensory oscillations (HFOs) found that CM and ictal EM both showed higher amplitudes after electrical stimuli followed by habituation, while interictal EM showed low amplitude at the initial assessment of HFO (Coppola et al.2013b). Sensory findings in this study are believed to be a result of the interconnections between the thalamus and the cortex. Hence, from these physiological studies, CM resembles an ongoing process of ictal EM or in other words, a “never-ending migraine attack” described by Schoenen (2011).
Central sensitization
In addition to its effect on peripheral trigeminal system, intense, repeated, and sustained noxious stimulation in trigeminal afferents can lead to the state of hyperexcitability of the central trigeminal neurons, resulting in central sensitization. In this state, central neurons display an increase in spontaneous activity, reduction in threshold for activation, and enlargement of nociceptive neuron receptive fields (Latremoliere and Woolf, 2009). Regarding episodic migraine, central sensitization accounts for scalp and forehead allodynia observed during the attack. Relationship between allodynia and chronic migraine is well established. The prevalence of cutaneous allodynia among patients with CM is higher than the estimates for episodic populations, ranging from 40 to 90% (Ashkenazi et al., 2007, Mathew et al., 2016). Allodynia is an independent predictor for the increase in number of migraine days and a risk factor for migraine chronification (Louter et al., 2013). This clinical presentation clearly illustrates that central sensitization of the trigeminal system is involved in the process of migraine chronification.
Activation of the glutamate N-methyl-D-aspartate (NMDA) receptors plays a crucial role in the development and central sensitization maintenance, possibly through the process of phosphorylation. The proposed pathway involves sustained CGRP release from central axons of trigeminal ganglionic neurons can trigger the activation of the mitogen-activated protein kinase (MAPK) signaling cascade. The activated kinase promotes the phosphorylation of the NR1 subunit of the NMDA receptor, causing changes in channel transduction and excitability of postsynaptic neurons. This CGRP-induced process facilitates nociceptive transmission and contributes to the development and maintenance of a sensitized, hyperresponsive state of the second-order pain transmission neurons within the central nervous system, thus contributing to central sensitization (Iyengar et al., 2017).
Activation of the MAPK signaling cascade also modifies the transcription factor controlling the genetic transcription process in the postsynaptic cells. This process leads to the shift of central sensitization from being activity-dependent to activity-independent and may indicate a mechanism driving the progression of EM to CM (Iyengar et al., 2019). The sensitization of trigeminothalamic neurons is likely to account for the widespread cutaneous allodynia involving extracephalic regions.
Medication overuse, sensitization, and endogenous brainstem modulating system
Overconsumption of acute medication is a common problem in patients with migraine. In addition to other adverse effects, it can lead to the deterioration of headache, causing medication overuse headache (MOH). Several epidemiologic studies have confirmed that medication overuse is a strong risk factor for the progression of migraine. For example, data from the AMPP study suggested that EM converts to CM at a rate of 2.5% per year (Bigal et al., 2008).
Furthermore, animal studies have clearly demonstrated that overconsumption of acute medication, either simple analgesic, opiates, or migraine specific agents, such as triptans and ergots, can alter the endogenous modulating process, resulting in trigeminal nociceptive facilitation. Chronic morphine intake in rats significantly increased the amplitude of monosynaptic excitatory postsynaptic current (EPSCs) in dorsal horn neurons evoked from the dorsal root and the frequency of spontaneous EPSCs in the spinal cord slice model. On the contrary, morphine treatment decreased the EPSCs, and NMDA currents evoked by direct puff NMDA application. These results indicate that chronic opioid treatment potentiates presynaptic, but impairs postsynaptic, NMDAR activity in the spinal cord (Zhao et al., 2012). Expansion of cutaneous receptive fields and lower thresholds of dura-sensitive medullary dorsal horn neurons were observed in rats receiving sustained infusion of morphine, indicating the presence of sensitization (Okada-Ogawa et al., 2009). Similar to the changes caused by morphine, chronic exposure to triptans also leads to sensitization. In rats, sustained administration of triptans elicited time-dependent and reversible cutaneous tactile allodynia along with increased expressions of CGRP in trigeminal dural afferents. These responses were maintained throughout and transiently after drug delivery. Interestingly, two weeks after triptan exposure, rats with normal sensory thresholds still showed enhanced cutaneous allodynia and increased CGRP in the blood following challenge with a nitric oxide donor, indicating the presence of latent sensitization (De Felice et al., 2010). The mechanism underlying triptan-induced nociceptor sensitization may include the deficit in PKA-mediated inhibition of nitric oxide–Nav1.9 coupling, resulting in the hyperactivity of meningeal nociceptors and inflammation in the meninges (Bonnet et al., 2019).
The sensitivity of the trigeminal nociceptive system depends on several factors including the descending control from the brainstem. Several studies have shown that chronic medication exposure can alter the endogenous serotonin (5-HT)-dependent brainstem modulating system. For example, chronic sumatriptan exposure induced significant increases in the 5-HT synthesis rate in many projection areas but had no effect in the dorsal raphe nucleus. The authors hypothesized that the 5-HT transporter upregulation might possibly result from a down-regulation/desensitization of 5-HT1 receptors and/or unmasking of excitatory triptan-sensitive 5-HT receptors. (Dobson et al., 2004). An increase of platelet 5-HT transporter activity was also demonstrated in patients with analgesic and triptan induced MOH (Ayzenberg et al 2008). Animals with low 5-HT and those with chronic medication exposure share physiological changes. Increased susceptibility to developing CSD and trigeminal nociceptive facilitation were seen in both conditions (Supornsilpchai et al., 2006, Supornsilpchai et al., 2010). Diminished function of the nucleus raphe magnus, a major 5-HTergic neuronal cell group in the medulla has been demonstrated in animals with chronic analgesics exposure. Inhibiting this brainstem nucleus in control animals significantly increased the frequency of CSD-evoked direct current shift and Fos-immunoreactive neurons in the TNC, reflecting its inhibitory effect on cortical activity. This modulating effect was lost in animals with chronic acetaminophen exposure. The absence of nucleus raphe magnus effect was also evident in another animal model using intravenous systemic infusion of nitroglycerin (Potewiratnanond et al., 2019).
It is known that medication overuse causes deterioration of headache only in patients with preexisting primary headaches disorders, especially migraine. This observation implies that baseline activation of trigeminal nociceptive pathways is a necessary factor for medication-induced headache transformation. It should be noted that not all types of trigeminal nociception can lead to MOH. MOH usually develops in the setting of headaches with an inflammatory component, such as migraine. This clinical observation implies that inflammatory nociception may be required in the process of transformation. In a rat model, chemically induced meningeal nociception plus chronic rizatriptan exposure induced nociception-related behaviors in rats and increased Fos expression in the cerebral cortex and trigeminovascular pathway, whereas chronic rizatriptan exposure alone did not show Fos expression. In addition, compared with IS alone, IS plus rizatriptan showed a more robust Fos expression (Su et al.2016). Nation et al showed that morphine-primed and high-dose sumatriptan-primed rats demonstrated a loss of diffuse noxious inhibitory control on day 21 only if they received a capsaicin injection on day 7. These findings suggest that migraine medications combined with repeated episodes of inflammatory pain, are required to produce long-lasting alterations in descending pain modulation (Nation et al 2019).
Evidence has shown that up-regulation of CGRP may be an important factor contributing to the development of MOH. An in vitro experiment demonstrated increased expression of CGRP and substance P in cultured dorsal root ganglia after repetitive morphine exposure (Ma et al 2000). Bright light stress and NO donor challenge produced a long-lasting cutaneous allodynia and significantly increased plasma CGRP levels in animals with chronic sumatriptan exposure. The evoked cutaneous allodynia can be inhibited by fremanezumab, a fully humanized CGRP antibody. The authors suggest that acute migraine medications may promote MOH in susceptible individuals through CGRP-dependent mechanisms and that anti-CGRP antibodies may be a useful clinical strategy for MOH treatment (Kopruszinski et al 2017).
Taken together, chronic acute medication exposure with coexisting inflammatory nociception, can alter the descending endogenous 5-HT modulating system and upregulate CGRP, causing long-lasting sensitization in trigeminal nociceptive pathway. The dysregulation of descending pain modulatory circuits results in a net descending facilitation and promotes pain chronification.
Role of the hypothalamus and cerebral cortex
As mentioned in the functional imaging section, change in hypothalamic function has been demonstrated in patients with CM. A recent resting-state functional MRI study showed that, in patients with CM, the hypothalamus is more easily activated by external stimuli; additionally, it is strongly connected to the pain matrix in patients with CM. Brain connectivity between the pain matrix and serotonergic system in patients with CM is relatively weak. This imbalance may contribute to migraine chronification (Lee et al 2019).
The association between changes in brain networks and migraine chronification has been demonstrated in an animal model of CM. Application of inflammatory soup in awake, fully conscious, rats, enhanced thalamic, hypothalamic, hippocampal, and somatosensory cortex responses to mechanical stimulation of the face. Resting state MRI data revealed altered functional connectivity in the default mode, sensorimotor, interoceptive (salience) and autonomic networks. These findings suggest that activation and sensitization of meningeal nociceptors results in several adaptive responses including modifying hypothalamic regulation of autonomic outflow to the cranium (Becerra et al 2017). Global network disruption has been identified in both EM and CM, as indicated by the highly segregated network in migraine patients compared to non-headache controls. Higher modularity but a lower clustering coefficient in CM is suggestive of more segregation in this group compared to EM. The presence of a segregated network could be a sign of maladaptive reorganization of headache related brain circuits, leading to migraine attacks or secondary alterations to pain (Michels et al 2021). The wide range of changes in brain networks may also explain the comorbidities, such as depression and various types of chronic painful syndromes, frequently seen in patients with CM.
Migraine progression – Proposed hypothesis
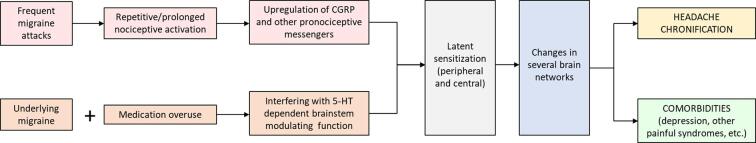
The above information shows that on-going trigeminal nociception, especially induced by CGRP-related neurogenic inflammation, is required for migraine progression. As suggested by Edvinsson et al, continued stimulation of C fibers during repeated migraine attacks, and the ensuing activation of Aδ fibers and satellite ganglion cells, leads to neurogenic neuroinflammation in the trigeminovascular system, thereby promoting the chronification process (Edvinsson et al., 2019). Repetitive and prolonged inflammatory nociception will lead to peripheral and then central sensitization, resulting in changes in several brain networks related to both pain and non-pain behaviours. The process of CGRP-dependent inflammatory nociception is further enhanced when the endogenous brainstem modulating systems, especially the 5-HT dependent ones, is impaired (Fig. 4). Chronic medication can alter 5-HT modulating system and upregulate CGRP, causing long-lasting sensitization in trigeminal nociceptive pathway. The dysregulation of descending pain modulatory circuits results in a net descending facilitation, lack of habituation and promotes pain chronification. Therefore, combination of chronic repetitive inflammatory nociception and diminished brainstem modulation is a possible mechanism contributing to migraine progression.
Fig. 4.
Mechanisms of migraine progression. Frequent migaine attacks result in the repetitive and prolonged inflammatory (CGRP-induced) nociception and induce peripheral and then central sensitization. The increase in trigeminal nociceptive sensitivity leads to adaptive changes in several brain networks related to both pain and non-pain behaviours. Chronic medication can alter the endogenous brainstem modulating systems, especially 5-HT dependent and render sensitization process. Combination between increased pain matrix connectivity including hypothalamic hyperactivity and weak 5-HTergic system may contribute to migraine chronification.
Funding
None.
Ethics approval
N/A.
Consent to participate:
N/A.
Consent for publication:
N/A.
Availability of data and material:
N/A.
Code availability
N/A.
CRediT authorship contribution statement
Wanakorn Rattanawong: Conceptualization, Writing - original draft, Writing - review & editing. Alan Rapoport: Writing - original draft, Writing - review & editing. Anan Srikiatkhachorn: Conceptualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to express our gratitude to Dr. Sranya Phaisawang for her help in the construction and language editing of the manuscript.
References
- Adams A.M., Serrano D., Buse D.C., Reed M.L., Marske V., Fanning K.M., Lipton R.B. The impact of chronic migraine: The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study methods and baseline results. Cephalalgia. 2015;35(7):563–578. doi: 10.1177/0333102414552532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameijeira P., Leira Y., Domínguez C., Leira R., Blanco J. Association between periodontitis and chronic migraine: a case-control study. Odontology. 2019;107(1):90–95. doi: 10.1007/s10266-018-0360-7. [DOI] [PubMed] [Google Scholar]
- Anapindi K., Yang N., Romanova E.V., Rubakhin S.S., Tipton A., Dripps I., Sheets Z., Sweedler J.V., Pradhan A.A. PACAP and Other Neuropeptide Targets Link Chronic Migraine and Opioid-induced Hyperalgesia in Mouse Models. Mol. Cell. Proteomics: MCP. 2019;18(12):2447–2458. doi: 10.1074/mcp.RA119.001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G.C., John M.T., Ohrbach R., Nixdorf D.R., Schiffman E.L., Truelove E.S., List T. Influence of headache frequency on clinical signs and symptoms of TMD in subjects with temple headache and TMD pain. Pain. 2011;152(4):765–771. doi: 10.1016/j.pain.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry–the methods. NeuroImage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashina S., Lyngberg A., Jensen R. Headache characteristics and chronification of migraine and tension-type headache: A population-based study. Cephalalgia. 2010;30(8):943–952. doi: 10.1177/0333102409357958. [DOI] [PubMed] [Google Scholar]
- Ashina S., Serrano D., Lipton R.B., Maizels M., Manack A.N., Turkel C.C., Reed M.L., Buse D.C. Depression and risk of transformation of episodic to chronic migraine. J. Headache Pain. 2012;13(8):615–624. doi: 10.1007/s10194-012-0479-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A., Sholtzow M., Shaw J.W., Burstein R., Young W.B. Identifying cutaneous allodynia in chronic migraine using a practical clinical method. Cephalalgia. 2007;27(2):111–117. doi: 10.1111/j.1468-2982.2006.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurora S.K. Spectrum of illness: understanding biological patterns and relationships in chronic migraine. Neurology. 2009;72(5 Suppl):S8–S13. doi: 10.1212/WNL.0b013e31819749fd. [DOI] [PubMed] [Google Scholar]
- Aurora S.K., Barrodale P.M., Tipton R.L., Khodavirdi A. Brainstem dysfunction in chronic migraine as evidenced by neurophysiological and positron emission tomography studies. Headache. 2007;47(7):996–1007. doi: 10.1111/j.1526-4610.2007.00853.x. [DOI] [PubMed] [Google Scholar]
- Aurora S.K., Brin M.F. Chronic Migraine: An Update on Physiology, Imaging, and the Mechanism of Action of Two Available Pharmacologic Therapies. Headache. 2017;57(1):109–125. doi: 10.1111/head.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayzenberg I., Obermann M., Leineweber K., Franke L., Yoon M.S., Diener H.C., Katsarava Z. Increased activity of serotonin uptake in platelets in medication overuse headache following regular intake of analgesics and triptans. J. Headache Pain. 2008;9(2):109–112. doi: 10.1007/s10194-008-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L., Bishop J., Barmettler G., Kainz V., Burstein R., Borsook D. Brain network alterations in the inflammatory soup animal model of migraine. Brain Res. 2017;1660:36–46. doi: 10.1016/j.brainres.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal M.E., Lipton R.B. Migraine at all ages. Curr. Pain Headache Rep. 2006;10(3):207–213. doi: 10.1007/s11916-006-0047-6. [DOI] [PubMed] [Google Scholar]
- Bigal M.E., Lipton R.B. Clinical course in migraine: conceptualizing migraine transformation. Neurology. 2008;71(11):848–855. doi: 10.1212/01.wnl.0000325565.63526.d2. [DOI] [PubMed] [Google Scholar]
- Bigal M.E., Lipton R.B. The prognosis of migraine. Curr. Opin. Neurol. 2008;21(3):301–308. doi: 10.1097/WCO.0b013e328300c6f5. [DOI] [PubMed] [Google Scholar]
- Bigal M.E., Serrano D., Buse D., Scher A., Stewart W.F., Lipton R.B. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48(8):1157–1168. doi: 10.1111/j.1526-4610.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- Bilgiç B., Kocaman G., Arslan A.B., Noyan H., Sherifov R., Alkan A., Asil T., Parman Y., Baykan B. Volumetric differences suggest involvement of cerebellum and brainstem in chronic migraine. Cephalalgia. 2016;36(4):301–308. doi: 10.1177/0333102415588328. [DOI] [PubMed] [Google Scholar]
- Blumenfeld A.M., Varon S.F., Wilcox T.K., Buse D.C., Kawata A.K., Manack A., Goadsby P.J., Lipton R.B. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS) Cephalalgia. 2011;31(3):301–315. doi: 10.1177/0333102410381145. [DOI] [PubMed] [Google Scholar]
- Bonnet C., Hao J., Osorio N., Donnet A., Penalba V., Ruel J., Delmas P. Maladaptive activation of Nav1.9 channels by nitric oxide causes triptan-induced medication overuse headache. Nat. Commun. 2019;10(1):4253. doi: 10.1038/s41467-019-12197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N., Schultz L.R., Stewart W.F., Lipton R.B., Lucia V.C., Welch K.M. Headache and major depression: is the association specific to migraine? Neurology. 2000;54(2):308–313. doi: 10.1212/wnl.54.2.308. [DOI] [PubMed] [Google Scholar]
- Burstein R., Noseda R., Borsook D. Migraine: multiple processes, complex pathophysiology. J. Neurosci. 2015;35(17):6619–6629. doi: 10.1523/JNEUROSCI.0373-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse D.C., Manack A., Serrano D., Turkel C., Lipton R.B. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J. Neurol. Neurosurg. Psychiatry. 2010;81(4):428–432. doi: 10.1136/jnnp.2009.192492. [DOI] [PubMed] [Google Scholar]
- Buse D.C., Rains J.C., Pavlovic J.M., Fanning K.M., Reed M.L., Manack Adams A., Lipton R.B. Sleep Disorders Among People With Migraine: Results From the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache. 2019;59(1):32–45. doi: 10.1111/head.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse D.C., Reed M.L., Fanning K.M., Bostic R., Dodick D.W., Schwedt T.J., Munjal S., Singh P., Lipton R.B. Comorbid and co-occurring conditions in migraine and associated risk of increasing headache pain intensity and headache frequency: results of the migraine in America symptoms and treatment (MAST) study. J. Headache Pain. 2020;21(1):23. doi: 10.1186/s10194-020-1084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun A.H., Ford S., Millen C., Finkel A.G., Truong Y., Nie Y. The prevalence of neck pain in migraine. Headache. 2010;50(8):1273–1277. doi: 10.1111/j.1526-4610.2009.01608.x. [DOI] [PubMed] [Google Scholar]
- Cernuda-Morollón E., Larrosa D., Ramón C., Vega J., Martínez-Camblor P., Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology. 2013;81(14):1191–1196. doi: 10.1212/WNL.0b013e3182a6cb72. [DOI] [PubMed] [Google Scholar]
- Cernuda-Morollón E., Martínez-Camblor P., Ramón C., Larrosa D., Serrano-Pertierra E., Pascual J. CGRP and VIP levels as predictors of efficacy of Onabotulinumtoxin type A in chronic migraine. Headache. 2014;54(6):987–995. doi: 10.1111/head.12372. [DOI] [PubMed] [Google Scholar]
- Chalmer M.A., Hansen T.F., Lebedeva E.R., Dodick D.W., Lipton R.B., Olesen J. Proposed new diagnostic criteria for chronic migraine. Cephalalgia. 2020;40(4):399–406. doi: 10.1177/0333102419877171. [DOI] [PubMed] [Google Scholar]
- Chen Z., Chen X., Liu M., Ma L., Yu S. Volume of Hypothalamus as a Diagnostic Biomarker of Chronic Migraine. Front. Neurol. 2019;10:606. doi: 10.3389/fneur.2019.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C., Tang C.H., Ng K., Wang S.J. Comorbidity profiles of chronic migraine sufferers in a national database in Taiwan. J. Headache Pain. 2012;13(4):311–319. doi: 10.1007/s10194-012-0447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola G. Neural plasticity and migraine. J. Headache Pain. 2015;16(Suppl 1):A26. doi: 10.1186/1129-2377-16-S1-A26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola G., Di Lorenzo C., Schoenen J., Pierelli F. Habituation and sensitization in primary headaches. J. Headache Pain. 2013;14(1):65. doi: 10.1186/1129-2377-14-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola G., Iacovelli E., Bracaglia M., Serrao M., Di Lorenzo C., Pierelli F. Electrophysiological correlates of episodic migraine chronification: evidence for thalamic involvement. J. Headache Pain. 2013;14(1):76. doi: 10.1186/1129-2377-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola G., Petolicchio B., Di Renzo A., Tinelli E., Di Lorenzo C., Parisi V., Serrao M., Calistri V., Tardioli S., Cartocci G., Ambrosini A., Caramia F., Di Piero V., Pierelli F. Cerebral gray matter volume in patients with chronic migraine: correlations with clinical features. J. Headache Pain. 2017;18(1):115. doi: 10.1186/s10194-017-0825-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta G., Colasanti A., Rabiner E.A., Gunn R.N., Malik O., Ciccarelli O., Nicholas R., Van Vlierberghe E., Van Hecke W., Searle G., Santos-Ribeiro A., Matthews P.M. Neuroinflammation and its relationship to changes in brain volume and white matter lesions in multiple sclerosis. Brain. 2017;140(11):2927–2938. doi: 10.1093/brain/awx228. [DOI] [PubMed] [Google Scholar]
- De Felice M., Ossipov M.H., Wang R., Lai J., Chichorro J., Meng I., Dodick D.W., Vanderah T.W., Dussor G., Porreca F. Triptan-induced latent sensitization: a possible basis for medication overuse headache. Ann. Neurol. 2010;67(3):325–337. doi: 10.1002/ana.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tommaso M., Valeriani M., Guido M., Libro G., Specchio L.M., Tonali P., Puca F. Abnormal brain processing of cutaneous pain in patients with chronic migraine. Pain. 2003;101(1–2):25–32. doi: 10.1016/s0304-3959(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Di Clemente L., Coppola G., Magis D., Fumal A., De Pasqua V., Di Piero V., Schoenen J. Interictal habituation deficit of the nociceptive blink reflex: an endophenotypic marker for presymptomatic migraine? Brain. 2007;130(Pt 3):765–770. doi: 10.1093/brain/awl351. [DOI] [PubMed] [Google Scholar]
- Diener H.C., Marmura M.J., Tepper S.J., Cowan R., Starling A.J., Diamond M.L., Hirman J., Mehta L., Brevig T., Sperling B., Cady R. Efficacy, tolerability, and safety of eptinezumab in patients with a dual diagnosis of chronic migraine and medication-overuse headache: Subgroup analysis of PROMISE-2. Headache. 2021;61(1):125–136. doi: 10.1111/head.14036. [DOI] [PubMed] [Google Scholar]
- Dobson C.F., Tohyama Y., Diksic M., Hamel E. Effects of acute or chronic administration of anti-migraine drugs sumatriptan and zolmitriptan on serotonin synthesis in the rat brain. Cephalalgia. 2004;24(1):2–11. doi: 10.1111/j.1468-2982.2004.00647.x. [DOI] [PubMed] [Google Scholar]
- Dodick D.W. A phase-by-phase review of migraine pathophysiology. Headache. 2018;58(Suppl 1):4–16. doi: 10.1111/head.13300. [DOI] [PubMed] [Google Scholar]
- Dodick D.W., Doty E.G., Aurora S.K., Ruff D.D., Stauffer V.L., Jedynak J., Dong Y., Pearlman E.M. Medication overuse in a subgroup analysis of phase 3 placebo-controlled studies of galcanezumab in the prevention of episodic and chronic migraine. Cephalalgia. 2021;41(3):340–352. doi: 10.1177/0333102420966658. [DOI] [PubMed] [Google Scholar]
- Domínguez, C., López, A., Ramos-Cabrer, P., Vieites-Prado, A., Pérez-Mato, M., Villalba, C., Sobrino, T., Rodriguez-Osorio, X., Campos, F., Castillo, J., & Leira, R., 2019. Iron deposition in periaqueductal gray matter as a potential biomarker for chronic migraine. Neurology, 92(10), e1076–e1085. [DOI] [PubMed]
- Domínguez C., Vieites-Prado A., Pérez-Mato M., Sobrino T., Rodríguez-Osorio X., López A., Campos F., Martínez F., Castillo J., Leira R. Role of adipocytokines in the pathophysiology of migraine: a cross-sectional study. Cephalalgia. 2018;38(5):904–911. doi: 10.1177/0333102417720213. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Haanes K.A., Warfvinge K. Does inflammation have a role in migraine? Nat. Rev. Neurol. 2019;15(8):483–490. doi: 10.1038/s41582-019-0216-y. [DOI] [PubMed] [Google Scholar]
- Eikermann-Haerter K., Huang S.Y. White Matter Lesions in Migraine. Am. J. Pathol. 2021;191(11):1955–1962. doi: 10.1016/j.ajpath.2021.02.007. [DOI] [PubMed] [Google Scholar]
- Goadsby P.J., Edvinsson L., Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990;28(2):183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- Han, L., Liu, Y., Xiong, H., & Hong, P., 2019. CGRP monoclonal antibody for preventive treatment of chronic migraine: An update of meta-analysis. Brain and behavior, 9(2), e01215. [DOI] [PMC free article] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. (2018). Cephalalgia : an international journal of headache, 38(1), 1–211. [DOI] [PubMed]
- Ishii R., Schwedt T.J., Dumkrieger G., Lalvani N., Craven A., Goadsby P.J., Lipton R.B., Olesen J., Silberstein S.D., Burish M.J., Dodick D.W. Chronic versus episodic migraine: The 15-day threshold does not adequately reflect substantial differences in disability across the full spectrum of headache frequency. Headache. 2021;61(7):992–1003. doi: 10.1111/head.14154. [DOI] [PubMed] [Google Scholar]
- Iyengar S., Ossipov M.H., Johnson K.W. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain. 2017;158(4):543–559. doi: 10.1097/j.pain.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S., Johnson K.W., Ossipov M.H., Aurora S.K. CGRP and the Trigeminal System in Migraine. Headache. 2019;59(5):659–681. doi: 10.1111/head.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M.U., Park J.W., Kho H.S., Chung S.C., Chung J.W. Plasma and saliva levels of nerve growth factor and neuropeptides in chronic migraine patients. Oral Dis. 2011;17(2):187–193. doi: 10.1111/j.1601-0825.2010.01717.x. [DOI] [PubMed] [Google Scholar]
- Kandel E.R., Tauc L. Heterosynaptic facilitation in neurones of the abdominal ganglion of Aplysia depilans. J. Physiol. 1965;181(1):1–27. doi: 10.1113/jphysiol.1965.sp007742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C.H., Wang S.J., Tsai C.F., Chen S.P., Wang Y.F., Fuh J.L. Psychiatric comorbidities in allodynic migraineurs. Cephalalgia. 2014;34(3):211–218. doi: 10.1177/0333102413505238. [DOI] [PubMed] [Google Scholar]
- Katsarava Z., Buse D.C., Manack A.N., Lipton R.B. Defining the differences between episodic migraine and chronic migraine. Curr. Pain Headache Rep. 2012;16(1):86–92. doi: 10.1007/s11916-011-0233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., Tu P.C., Zyloney C., Su T.P. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav. Brain Res. 2010;211(2):215–219. doi: 10.1016/j.bbr.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopruszinski C.M., Xie J.Y., Eyde N.M., Remeniuk B., Walter S., Stratton J., Bigal M., Chichorro J.G., Dodick D., Porreca F. Prevention of stress- or nitric oxide donor-induced medication overuse headache by a calcitonin gene-related peptide antibody in rodents. Cephalalgia. 2017;37(6):560–570. doi: 10.1177/0333102416650702. [DOI] [PubMed] [Google Scholar]
- Kropp P., Gerber W.D. Contingent negative variation during migraine attack and interval: evidence for normalization of slow cortical potentials during the attack. Cephalalgia. 1995;15(2):123–179. doi: 10.1046/j.1468-2982.1995.015002123.x. [DOI] [PubMed] [Google Scholar]
- Kruit M.C., van Buchem M.A., Hofman P.A., Bakkers J.T., Terwindt G.M., Ferrari M.D., Launer L.J. Migraine as a risk factor for subclinical brain lesions. JAMA. 2004;291(4):427–434. doi: 10.1001/jama.291.4.427. [DOI] [PubMed] [Google Scholar]
- Kruit M.C., van Buchem M.A., Launer L.J., Terwindt G.M., Ferrari M.D. Migraine is associated with an increased risk of deep white matter lesions, subclinical posterior circulation infarcts and brain iron accumulation: the population-based MRI CAMERA study. Cephalalgia. 2010;30(2):129–136. doi: 10.1111/j.1468-2982.2009.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai T.H., Chou K.H., Fuh J.L., Lee P.L., Kung Y.C., Lin C.P., Wang S.J. Gray matter changes related to medication overuse in patients with chronic migraine. Cephalalgia. 2016;36(14):1324–1333. doi: 10.1177/0333102416630593. [DOI] [PubMed] [Google Scholar]
- Latremoliere A., Woolf C.J. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J. Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.J., Park B.Y., Cho S., Kim S.T., Park H., Chung C.S. Increased connectivity of pain matrix in chronic migraine: a resting-state functional MRI study. J. Headache Pain. 2019;20(1):29. doi: 10.1186/s10194-019-0986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennerz J.K., Rühle V., Ceppa E.P., Neuhuber W.L., Bunnett N.W., Grady E.F., Messlinger K. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J. Comp. Neurol. 2008;507(3):1277–1299. doi: 10.1002/cne.21607. [DOI] [PubMed] [Google Scholar]
- Lerebours F., Boulanouar K., Barège M., Denuelle M., Bonneville F., Payoux P., Larrue V., Fabre N. Functional connectivity of hypothalamus in chronic migraine with medication overuse. Cephalalgia. 2019;39(7):892–899. doi: 10.1177/0333102419833087. [DOI] [PubMed] [Google Scholar]
- Lipton R.B., Bigal M.E., Diamond M., Freitag F., Reed M.L., Stewart W.F. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- Lipton R.B., Bigal M.E., Ashina S., Burstein R., Silberstein S., Reed M.L., Serrano D., Stewart W.F. Cutaneous allodynia in the migraine population. Ann. Neurol. 2008;63(2):148–158. doi: 10.1002/ana.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton R.B., Buse D.C., Saiers J., Fanning K.M., Serrano D., Reed M.L. Frequency and burden of headache-related nausea: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2013;53(1):93–103. doi: 10.1111/j.1526-4610.2012.02292.x. [DOI] [PubMed] [Google Scholar]
- Lipton R.B., Serrano D., Pavlovic J.M., Manack A.N., Reed M.L., Turkel C.C., Buse D.C. Improving the classification of migraine subtypes: an empirical approach based on factor mixture models in the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2014;54(5):830–849. doi: 10.1111/head.12332. [DOI] [PubMed] [Google Scholar]
- Lipton R.B., Cohen J.M., Bibeau K., Galic M., Seminerio M.J., Ramirez Campos V., Halker Singh R.B., Ailani J. Reversion From Chronic Migraine to Episodic Migraine in Patients Treated With Fremanezumab: Post Hoc Analysis From HALO CM Study. Headache. 2020;60(10):2444–2453. doi: 10.1111/head.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton R.B., Tepper S.J., Silberstein S.D., Kudrow D., Ashina M., Reuter U., Dodick D.W., Zhang F., Rippon G.A., Cheng S., Mikol D.D. Reversion from chronic migraine to episodic migraine following treatment with erenumab: Results of a post-hoc analysis of a randomized, 12-week, double-blind study and a 52-week, open-label extension. Cephalalgia. 2021;41(1):6–16. doi: 10.1177/0333102420973994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.Y., Chou K.H., Lee P.L., Fuh J.L., Niddam D.M., Lai K.L., Hsiao F.J., Lin Y.Y., Chen W.T., Wang S.J., Lin C.P. Hippocampus and amygdala volume in relation to migraine frequency and prognosis. Cephalalgia. 2017;37(14):1329–1336. doi: 10.1177/0333102416678624. [DOI] [PubMed] [Google Scholar]
- Louter M.A., Bosker J.E., van Oosterhout W.P., van Zwet E.W., Zitman F.G., Ferrari M.D., Terwindt G.M. Cutaneous allodynia as a predictor of migraine chronification. Brain. 2013;136(Pt 11):3489–3496. doi: 10.1093/brain/awt251. [DOI] [PubMed] [Google Scholar]
- Ma W., Zheng W.H., Kar S., Quirion R. Morphine treatment induced calcitonin gene-related peptide and substance P increases in cultured dorsal root ganglion neurons. Neuroscience. 2000;99(3):529–539. doi: 10.1016/s0306-4522(00)00226-8. [DOI] [PubMed] [Google Scholar]
- Mainero C., Boshyan J., Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann. Neurol. 2011;70(5):838–845. doi: 10.1002/ana.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manack A., Buse D.C., Serrano D., Turkel C.C., Lipton R.B. Rates, predictors, and consequences of remission from chronic migraine to episodic migraine. Neurology. 2011;76(8):711–718. doi: 10.1212/WNL.0b013e31820d8af2. [DOI] [PubMed] [Google Scholar]
- Maniyar F.H., Goadsby P.J. Functional imaging in chronic migraine. Curr. Pain Headache Rep. 2013;17(5):333. doi: 10.1007/s11916-013-0333-z. [DOI] [PubMed] [Google Scholar]
- Matharu M.S., Bartsch T., Ward N., Frackowiak R.S., Weiner R., Goadsby P.J. Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain. 2004;127(Pt 1):220–230. doi: 10.1093/brain/awh022. [DOI] [PubMed] [Google Scholar]
- Mathew P.G., Cutrer F.M., Garza I. A touchy subject: an assessment of cutaneous allodynia in a chronic migraine population. J. Pain Res. 2016;9:101–104. doi: 10.2147/JPR.S103238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A., Schulte L.H. Chronic migraine: risk factors, mechanisms and treatment. Nature Rev. Neurol. 2016;12(8):455–464. doi: 10.1038/nrneurol.2016.93. [DOI] [PubMed] [Google Scholar]
- Messlinger K., Balcziak L.K., Russo A.F. Cross-talk signaling in the trigeminal ganglion: role of neuropeptides and other mediators. J. Neural. Transm. 2020;127(4):431–444. doi: 10.1007/s00702-020-02161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels L., Koirala N., Groppa S., Luechinger R., Gantenbein A.R., Sandor P.S., Kollias S., Riederer F., Muthuraman M. Structural brain network characteristics in patients with episodic and chronic migraine. J. Headache Pain. 2021;22(1):8. doi: 10.1186/s10194-021-01216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation K.M., Dodick D.W., Navratilova E., Porreca F. Sustained exposure to acute migraine medications combined with repeated noxious stimulation dysregulates descending pain modulatory circuits: Relevance to medication overuse headache. Cephalalgia. 2019;39(5):617–625. doi: 10.1177/0333102418804157. [DOI] [PubMed] [Google Scholar]
- Neeb L., Bastian K., Villringer K., Israel H., Reuter U., Fiebach J.B. Structural gray matter alterations in chronic migraine: implications for a progressive disease? Headache. 2017;57(3):400–416. doi: 10.1111/head.13012. [DOI] [PubMed] [Google Scholar]
- Negm M., Housseini A.M., Abdelfatah M., Asran A. Relation between migraine pattern and white matter hyperintensities in brain magnetic resonance imaging. Egypt. J. Neurol., Psychiatry Neurosurg. 2018;54(1):24. doi: 10.1186/s41983-018-0027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro A., Sciattella P., Rossi D., Guglielmetti M., Martelletti P., Mennini F.S. Cost of chronic and episodic migraine patients in continuous treatment for two years in a tertiary level heada-che Centre. J. Headache Pain. 2019;20(1):120. doi: 10.1186/s10194-019-1068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niddam D.M., Lai K.L., Tsai S.Y., Lin Y.R., Chen W.T., Fuh J.L., Wang S.J. Neurochemical changes in the medial wall of the brain in chronic migraine. Brain. 2018;141(2):377–390. doi: 10.1093/brain/awx331. [DOI] [PubMed] [Google Scholar]
- Okada-Ogawa A., Porreca F., Meng I.D. Sustained morphine-induced sensitization and loss of diffuse noxious inhibitory controls in dura-sensitive medullary dorsal horn neurons. J. Neurosci. 2009;29(50):15828–15835. doi: 10.1523/JNEUROSCI.3623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J., Friberg L., Olsen T.S., Iversen H.K., Lassen N.A., Andersen A.R., Karle A. Timing and topography of cerebral blood flow, aura, and headache during migraine attacks. Ann. Neurol. 1990;28(6):791–798. doi: 10.1002/ana.410280610. [DOI] [PubMed] [Google Scholar]
- Ornello R., Ripa P., Pistoia F., Degan D., Tiseo C., Carolei A., Sacco S. Migraine and body mass index categories: a systematic review and meta-analysis of observational studies. J. Headache Pain. 2015;16:27. doi: 10.1186/s10194-015-0510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm-Meinders I.H., Koppen H., Terwindt G.M., Launer L.J., Konishi J., Moonen J.M., Bakkers J.T., Hofman P.A., van Lew B., Middelkoop H.A., van Buchem M.A., Ferrari M.D., Kruit M.C. Structural brain changes in migraine. JAMA. 2012;308(18):1889–1897. doi: 10.1001/jama.2012.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pereda S., Toriello-Suárez M., Ocejo-Vinyals G., Guiral-Foz S., Castillo-Obeso J., Montes-Gómez S., Martínez-Nieto R.M., Iglesias F., González-Quintanilla V., Oterino A. Serum CGRP, VIP, and PACAP usefulness in migraine: a case-control study in chronic migraine patients in real clinical practice. Mol. Biol. Rep. 2020;47(9):7125–7138. doi: 10.1007/s11033-020-05781-0. [DOI] [PubMed] [Google Scholar]
- Peterlin, B. L., Sacco, S., Bernecker, C., & Scher, A. I., 2016. Adipokines and Migraine: A Systematic Review. Headache, 56(4), 622–644. [DOI] [PMC free article] [PubMed]
- Pinheiro, C. F., Moreira, J. R., Carvalho, G. F., Zorzin, L., Dach, F., & Bevilaqua-Grossi, D., 2021. Interictal Photophobia and Phonophobia Are Related to the Presence of Aura and High Frequency of Attacks in Patients with Migraine. Applied Sciences, 11(6), 2474. MDPI AG.
- Plesh O., Adams S.H., Gansky S.A. Self-reported comorbid pains in severe headaches or migraines in a US national sample. Headache. 2012;52(6):946–956. doi: 10.1111/j.1526-4610.2012.02155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potewiratnanond P., le Grand S.M., Srikiatkhachorn A., Supronsinchai W. Altered activity in the nucleus raphe magnus underlies cortical hyperexcitability and facilitates trigeminal nociception in a rat model of medication overuse headache. BMC Neurosci. 2019;20(1):54. doi: 10.1186/s12868-019-0536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M.L., Fanning K.M., Serrano D., Buse D.C., Lipton R.B. Persistent frequent nausea is associated with progression to chronic migraine: AMPP study results. Headache. 2015;55(1):76–87. doi: 10.1111/head.12450. [DOI] [PubMed] [Google Scholar]
- Scher A.I., Stewart W.F., Ricci J.A., Lipton R.B. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain. 2003;106(1–2):81–89. doi: 10.1016/s0304-3959(03)00293-8. [DOI] [PubMed] [Google Scholar]
- Scher A.I., Stewart W.F., Lipton R.B. Caffeine as a risk factor for chronic daily headache: a population-based study. Neurology. 2004;63(11):2022–2027. doi: 10.1212/01.wnl.0000145760.37852.ed. [DOI] [PubMed] [Google Scholar]
- Schoenen J. Is chronic migraine a never-ending migraine attack? Pain. 2011;152(2):239–240. doi: 10.1016/j.pain.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Schulte L.H., Allers A., May A. Hypothalamus as a mediator of chronic migraine: Evidence from high-resolution fMRI. Neurology. 2017;88(21):2011–2016. doi: 10.1212/WNL.0000000000003963. [DOI] [PubMed] [Google Scholar]
- Serrano D., Lipton R.B., Scher A.I., Reed M.L., Stewart W., Adams A.M., Buse D.C. Fluctuations in episodic and chronic migraine status over the course of 1 year: implications for diagnosis, treatment and clinical trial design. J. Headache Pain. 2017;18(1):101. doi: 10.1186/s10194-017-0787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheftell F.D., Atlas S.J. Migraine and psychiatric comorbidity: from theory and hypotheses to clinical application. Headache. 2002;42(9):934–944. doi: 10.1046/j.1526-4610.2002.02217.x. [DOI] [PubMed] [Google Scholar]
- Silberstein S., Loder E., Diamond S., Reed M.L., Bigal M.E., Lipton R.B. Probable migraine in the United States: results of the American Migraine Prevalence and Prevention (AMPP) study. Cephalalgia. 2007;27(3):220–229. doi: 10.1111/j.1468-2982.2006.01275.x. [DOI] [PubMed] [Google Scholar]
- Sohn J.H., Kim C.H., Choi H.C. Differences in central facilitation between episodic and chronic migraineurs in nociceptive-specific trigeminal pathways. J. Headache Pain. 2016;17:35. doi: 10.1186/s10194-016-0637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, M., & Yu, S., 2018. Chronic migraine: A process of dysmodulation and sensitization. Molecular pain, 14, 1744806918767697. [DOI] [PMC free article] [PubMed]
- Su M., Ran Y.e., Han X., Liu Y., Zhang X.u., Tan Q., Li R., Yu S., Bolam P. Rizatriptan overuse promotes hyperalgesia induced by dural inflammatory stimulation in rats by modulation of the serotonin system. Eur. J. Neurosci. 2016;44(4):2129–2138. doi: 10.1111/ejn.13296. [DOI] [PubMed] [Google Scholar]
- Sun-Edelstein C., Rapoport A.M., Rattanawong W., Srikiatkhachorn A. The Evolution of Medication Overuse Headache: History. Pathophysiology and Clinical Update. CNS Drugs. 2021;35(5):545–565. doi: 10.1007/s40263-021-00818-9. [DOI] [PubMed] [Google Scholar]
- Supornsilpchai W., Sanguanrangsirikul S., Maneesri S., Srikiatkhachorn A. Serotonin depletion, cortical spreading depression, and trigeminal nociception. Headache. 2006;46(1):34–39. doi: 10.1111/j.1526-4610.2006.00310.x. [DOI] [PubMed] [Google Scholar]
- Supornsilpchai W., le Grand S.M., Srikiatkhachorn A. Cortical hyperexcitability and mechanism of medication-overuse headache. Cephalalgia. 2010;30(9):1101–1109. doi: 10.1177/0333102409355600. [DOI] [PubMed] [Google Scholar]
- Swartz R.H., Kern R.Z. Migraine is associated with magnetic resonance imaging white matter abnormalities: a meta-analysis. Arch. Neurol. 2004;61(9):1366–1368. doi: 10.1001/archneur.61.9.1366. [DOI] [PubMed] [Google Scholar]
- Torres-Ferrús M., Quintana M., Fernandez-Morales J., Alvarez-Sabin J., Pozo-Rosich P. When does chronic migraine strike? A clinical comparison of migraine according to the headache days suffered per month. Cephalalgia. 2017;37(2):104–113. doi: 10.1177/0333102416636055. [DOI] [PubMed] [Google Scholar]
- Unal Z., Domac F.M., Boylu E., Kocer A., Tanridag T., Us O. Blink reflex in migraine headache. Northern Clin. Istanbul. 2016;3(1):1–8. doi: 10.14744/nci.2016.30301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valfrè W., Rainero I., Bergui M., Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48(1):109–117. doi: 10.1111/j.1526-4610.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- Van Dongen R.M., Zielman R., Noga M., Dekkers O.M., Hankemeier T., van den Maagdenberg A.M., Terwindt G.M., Ferrari M.D. Migraine biomarkers in cerebrospinal fluid: A systematic review and meta-analysis. Cephalalgia. 2017;37(1):49–63. doi: 10.1177/0333102415625614. [DOI] [PubMed] [Google Scholar]
- Viana M., Bottiroli S., Sances G., Ghiotto N., Allena M., Guaschino E., Nappi G., Tassorelli C. Factors associated to chronic migraine with medication overuse: A cross-sectional study. Cephalalgia. 2018;38(14):2045–2057. doi: 10.1177/0333102418761047. [DOI] [PubMed] [Google Scholar]
- Weiller C., May A., Limmroth V., Jüptner M., Kaube H., Schayck R.V., Coenen H.H., Diener H.C. Brain stem activation in spontaneous human migraine attacks. Nat. Med. 1995;1(7):658–660. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- Welch K.M., Nagesh V., Aurora S.K., Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001;41(7):629–637. doi: 10.1046/j.1526-4610.2001.041007629.x. [DOI] [PubMed] [Google Scholar]
- Xu J., Kong F., Buse D.C. Predictors of episodic migraine transformation to chronic migraine: A systematic review and meta-analysis of observational cohort studies. Cephalalgia. 2020;40(5):503–516. doi: 10.1177/0333102419883355. [DOI] [PubMed] [Google Scholar]
- Yakubova A., Davidyuk Y., Tohka J., Khayrutdinova O., Kudryavtsev I., Nurkhametova D., Kamshilin A., Giniatullin R., Rizvanov A. Searching for Predictors of Migraine Chronification: a Pilot Study of 1911A>G Polymorphism of TRPV1 Gene in Episodic Versus Chronic Migraine. J. Mol. Neurosci.: MN. 2021;71(3):618–624. doi: 10.1007/s12031-020-01683-9. [DOI] [PubMed] [Google Scholar]
- Zhang M., Liu Y., Zhao M., Tang W., Wang X., Dong Z., Yu S. Depression and anxiety behaviour in a rat model of chronic migraine. J. Headache Pain. 2017;18(1):27. doi: 10.1186/s10194-017-0736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.L., Chen S.R., Chen H., Pan H.L. Chronic opioid potentiates presynaptic but impairs postsynaptic N-methyl-D-aspartic acid receptor activity in spinal cords: implications for opioid hyperalgesia and tolerance. J. Biol. Chem. 2012;287(30):25073–25085. doi: 10.1074/jbc.M112.378737. [DOI] [PMC free article] [PubMed] [Google Scholar]