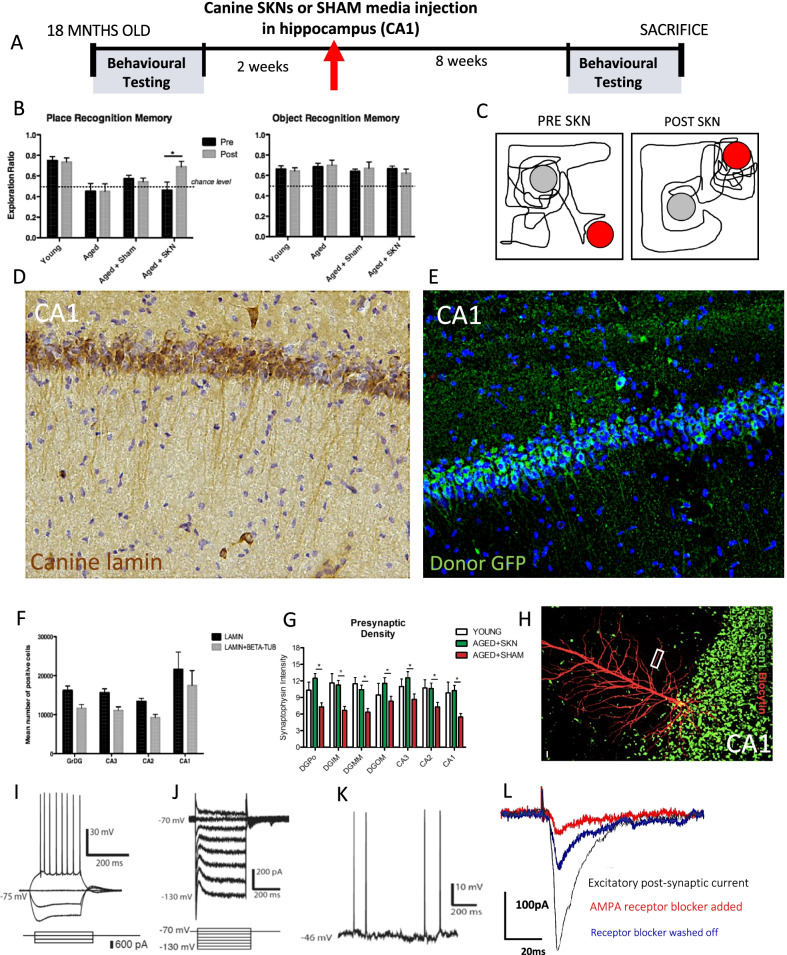
Fig. 4.
SKN neuronal engraftment and synaptic rescue in rodent model of age-related memory dysfunction. A Experimental paradigm. B Canine SKN cell transplantation rescues hippocampally-dependent Place Recognition Memory (PRM) back to young animal level (Time X Group F1,14 = 6.934, p = 0.02; left; see Additional file 1: Figure S24 for individual animal data) and has no effect on intact ORM (right). Untreated young and old animal data from our prior report [37]. C Exemplar trace of rodent exploration of familiar object in a novel position during PRM before and after treatment. D Canine-specific lamin marker of donor cells (see Additional file 1: Figures S15–17 for controls) shows extensive survival and engraftment of canine SKN cells in CA1 in main experiment (N = 16; see Additional file 1: Figure S18 for engraftment in other hippocampal areas). E Extension study (N = 2) with GFP-genetically labelled donor cells shows engraftment in CA1 with anatomically-correct cell bodies and penetrating dendrites; see Additional file 1: Figure S19 for co-expression with mature neuronal NeuN and neurofilament. F Stereological counts of donor cells throughout hippocampus marked by canine lamin, and Lamin + βIII tubulin co-expression indicative of in vivo neuronal differentiation (N = 16; see Additional file 1: Figure S19 for confocal fluorescent images). G Synaptophysin was restored to young animal levels throughout hippocampus compared to media vehicle (N = 16). H Donor cells genetically labelled with pZs-Green1 (green), allowing cell identification in a fresh hippocampal slice preparation, here filled with biocytin (red) to reveal pyramidal morphology including dendritic spines on primary dendrites (z-stack image, see Additional file 1: Figures S21–23 for orthogonal confocal images and high magnification of secondary dendrite with intense decoration by synaptic spines). I In a hippocampal slice preparation, SKN donor cells (k = 3) displayed elicited action potentials in CA1 under current clamp and J voltage clamp conditions. K Spontaneous action potentials in SKN donor cell in the dentate gyrus. L Excitatory post-synaptic currents in CA1 (same cell as in H) following stimulation of distal Schaffer collateral fibres, abolishable by AMPA-receptor blockade, indicating that canine SKN cells can integrate into physiological neuronal circuits. D, E and H are from independent animals