Fig. 2.
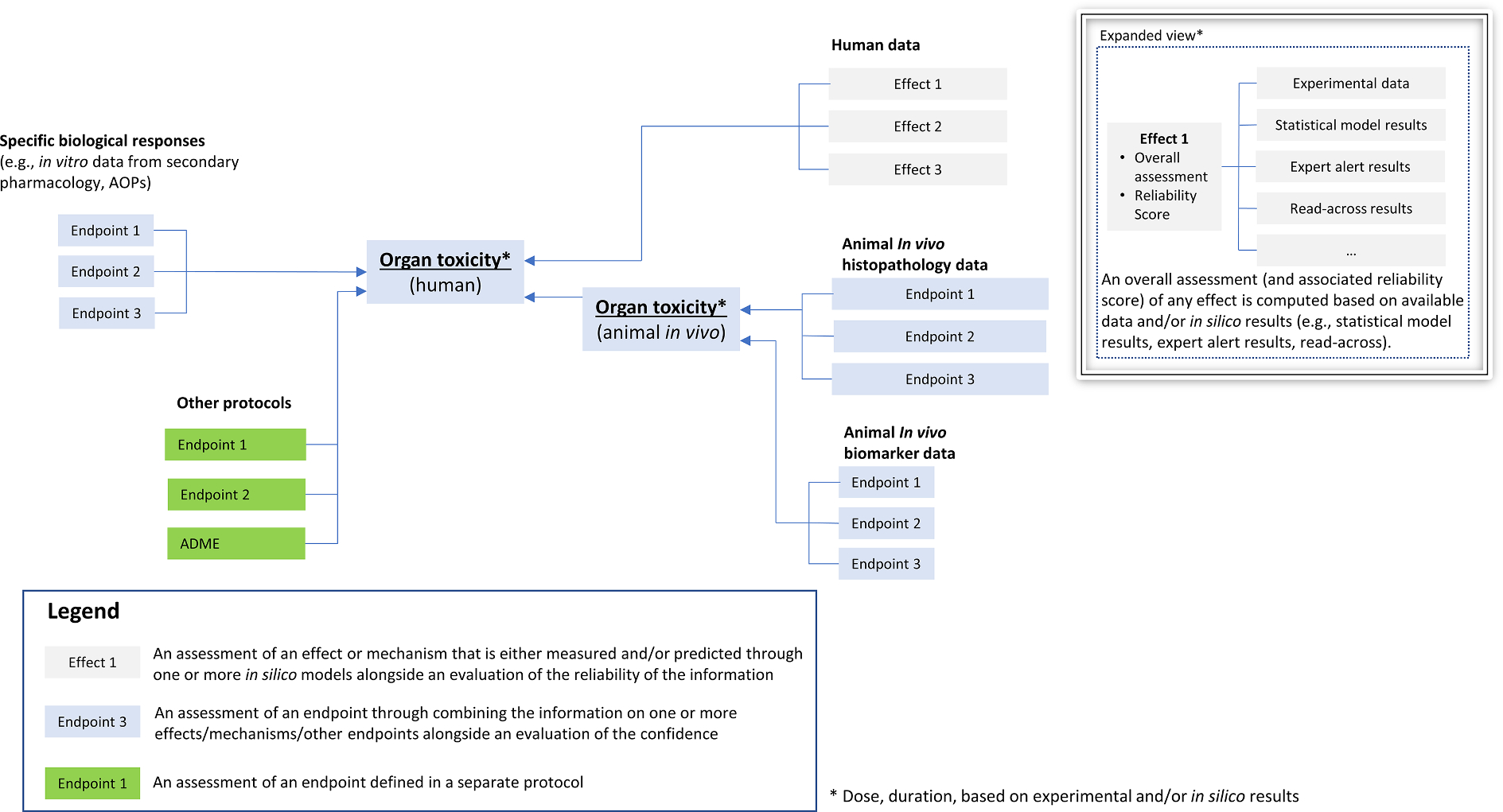
Draft outline of potential hazard assessment framework for organ toxicity (adapted from [8]). The draft framework combines information from in vitro approaches (e.g., biological responses from receptor-based assays), in vivo experiments, and human data. Other protocols (e.g., ADME or other organs) may feed a protocol for a given organ. Exposure scenarios (e.g., environmental, drug, consumer, accidental) may also be used to supplement the protocol. Effects (predicted by in silico methods or measured experimentally) are combined for the assessment of a given endpoint.
