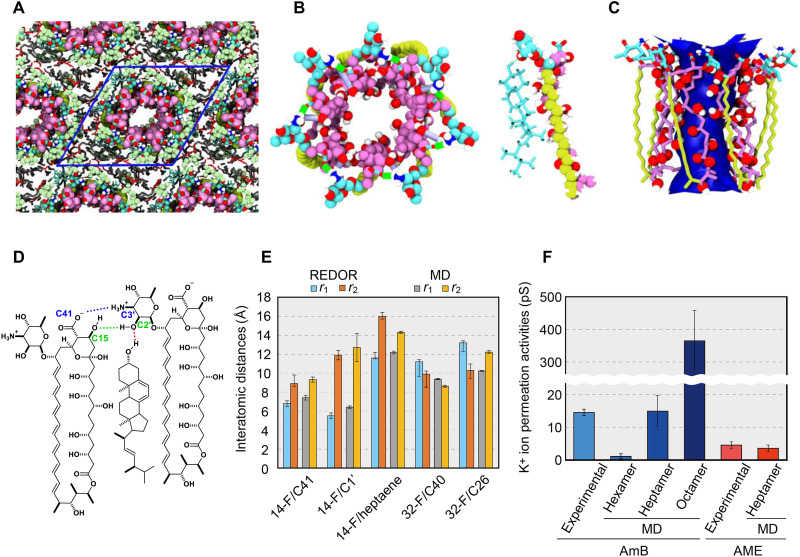
Fig. 4. Construction of asymmetric assembly and ion channel activity based on MD simulations.
(A) Initial structure of the AmB assemblies with periodic boundary conditions constructed on the basis of NMR results. (B) Snapshots of all-atom MD simulations of an AmB assembly without Erg (left) and an AmB-Erg complex (right). Bright blue shows the mycosamine moiety of AmB (both) and Erg (right). (C) Average structure of an AmB assembly showing the water-accessible region inside the pore. (D) Major intermolecular interactions stabilizing the AmB-Erg channel assembly (see figs. S29 and S38). (E) Interatomic distances measured by solid-state NMR experiments and the values from MD simulations. The distances differ between the front and back of the molecule and correspond to r1 and r2 (Fig. 3B); the structure obtained by MD is not rotationally symmetric, so the average ± SD are shown, where SD obtained from 10 runs of the simulation were less than 5%. (F) Experimental and MD-derived values of K+ permeability (30) (fig. S34 and table S12); the experiments in the literature carried out by using the membranes of DPhPC-Erg 5:1 (2 M KCl, at 100 mV) (30) and egg yolk lecithin (43). This clearly indicates that the channel that shows a value close to those of past single-channel measurements is the heptamer assembly.