Figure 2.
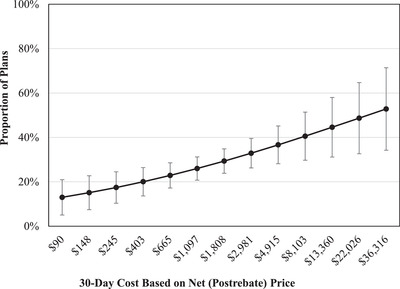
Association Between 30‐Day Drug Cost and Coverage in Part D Plan Formularies During the Year After FDA Approval for Drugs in Nonprotected Drug Classes
Results obtained from enrollment‐weighted multivariable logistic regression analysis, also controlling for plan type, approval year, FDA expedited approval, Orphan Drug Act designation, first‐in‐class status, pivotal trial design, and availability of added therapeutic benefit.
