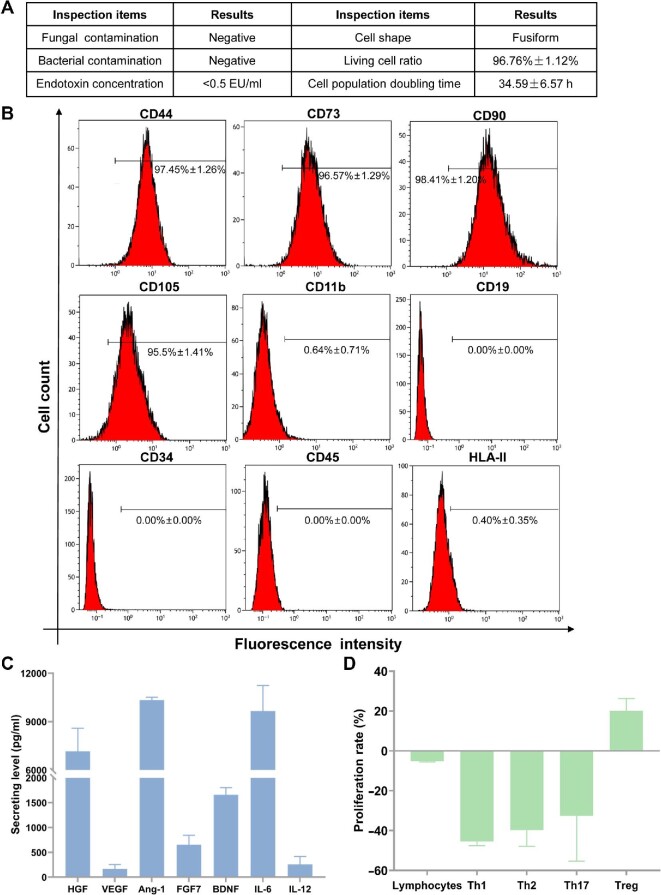
Figure 1.
Preclinical release inspections and efficacy assessment. (A) General biosafety and cell viability of hAMSCs were up to quality standard. (B) Release inspections on hAMSC surface antigens. (C) Analysis for cytokine secretions from hAMSC culture supernatants. (D) hAMSCs showed immunocompetence in vitro when cocultured with lymphocytes from healthy persons.