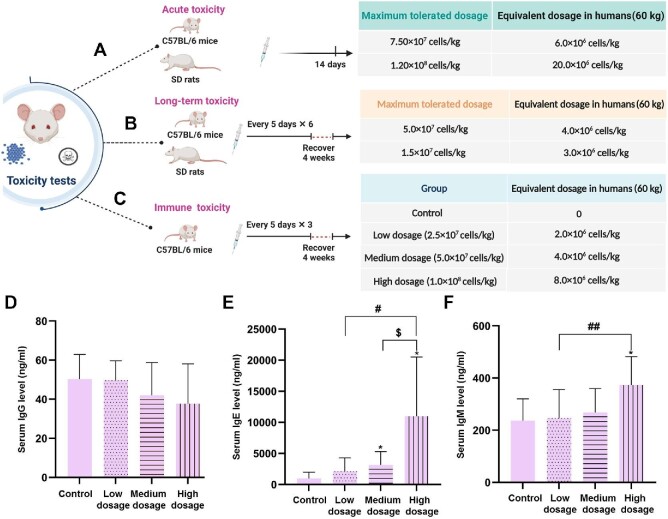
Figure 3.
Animal tests for maximum tolerated dosages and immune toxicity of hAMSCs. (A and B) Maximum tolerated hAMSC dosages in C57BL/6 mice and SD rats were investigated with acute (A) and long-term (B) toxicity tests. (C) Immune toxicity of hAMSCs in C57BL/6 mice was investigated in control, low-dosage, medium-dosage, and high-dosage groups. The estimated equivalent dosages in humans are listed. Created with BioRender.com. (D–F) Serum IgG (D), IgE (E), and IgM (F) levels were detected in immune toxicity tests. *P < 0.05 vs. control group;  P < 0.05 vs. medium-dosage group; #P < 0.05 and ##P < 0.01 vs. low-dosage group.
P < 0.05 vs. medium-dosage group; #P < 0.05 and ##P < 0.01 vs. low-dosage group.