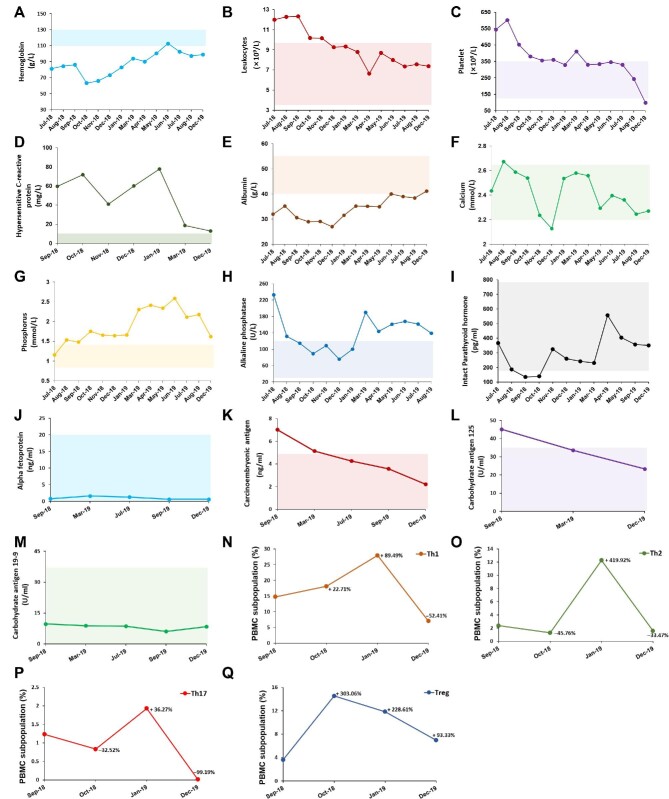
Figure 5.
The laboratory data of the uremic calciphylaxis patient before and after hAMSC treatment. (A–M) After applying hAMSC treatment, the levels of hemoglobin (A), leukocytes (B), platelet (C), hypersensitive C-reactive protein (D), serum albumin (E), serum calcium (F), serum phosphorus (G), serum alkaline phosphatase (H), serum intact parathyroid hormone (I), serum alpha fetoprotein (J), serum carcinoembryonic antigen (K), serum carbohydrate antigen 125 (L), and serum carbohydrate antigen 19-9 (M) were monitored. Shaded area shows the reference range of each indicator. The hemoglobin, calcium, phosphorus, and intact parathyroid hormone levels are based on the recommended ranges (KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease, 2012; Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Update Work Group, 2017), and others are based on the normal reference values of laboratory tests. (N–Q) PBMC subpopulations of the patient during the course of hAMSC treatment, including Th1 (N), Th2 (O), Th17 (P), and Treg (Q), were detected.