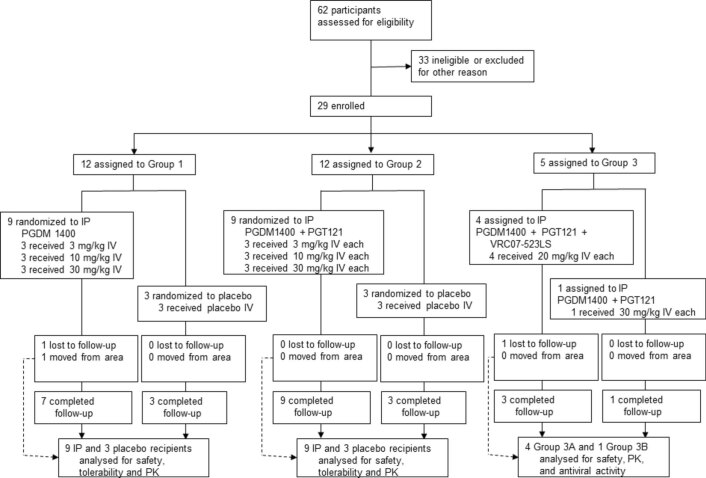
Extended Data Fig. 1. Participant enrollment and study design.
A total of 62 volunteers without and with HIV were screened for study participation. In individuals without HIV, PGDM1400 was sequentially administered alone or followed by PGT121 in a single IV infusion at increasing doses of 3 mg/kg (Groups 1A and 2A), 10 mg/kg (Groups 1B and 2B), or 30 mg/kg (Groups 1C and 2C) for each bNAb. Viremic participants with HIV (Group 3) received a single IV dose of PGDM1400, PGT121 and VRC07-523LS at 20 mg/kg each (Group 3A) or a single dose of PGDM1400 and PGT121 at 30 mg/kg each (Group 3B). Of a total of 62 subjects screened, 24 participants without HIV and 5 participants with HIV were enrolled. Of the 29 participants enrolled, 26 completed the study on the planned schedule, and three terminated the study early (one Group 1B participant and one Group 3A participant were lost to follow-up and one Group 1C participant moved out of the area). However, no participants were excluded from the safety analyses as all participants had accrued follow-up time after IP administration.