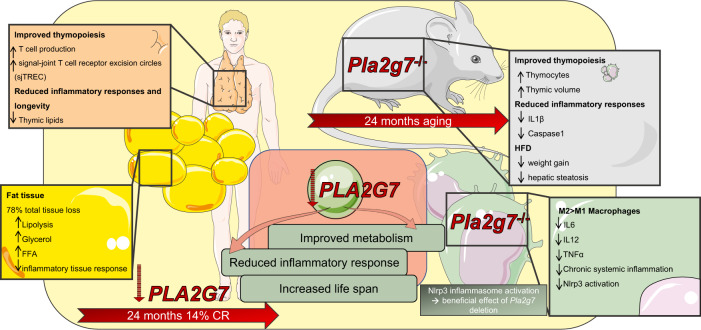
Fig. 1.
The Improvement of health and lower inflammation through Caloric Restriction (CR) via Pla2g7 downregulation. Improved thymopoiesis is found by enhanced T cell generation and greater levels of signal-joint T cell receptor excision circles (sjTREC) after a 14% CR for 24 months. Thymic lipid buildup after 24 months of CR was reduced in healthy mid-aged humans, which was used to estimate longevity. After CR, adipose tissue reduction accounted for 78% of overall weight loss. Increased lipolysis, as demonstrated by higher levels of glycerol and free fatty acids (FFA), helped to create an anti-inflammatory environment and improved adipose tissue metabolism. Downregulation of platelet activating factor acetyl hydrolase (PLA2G7) in humans experiencing CR is thought to be the origin of these effects, which mediates immune-metabolic effects that contribute to lower inflammation and a longer lifespan. The CRISPR Cas technique was used to generate Pla2g7−/− knockout mice, which were protected from weight gain and steatosis when fed a high-fat diet (HFD). Thymocyte production and thymic volume were raised in these animals, highlighting the regulatory effects of PLA2G7 in the human cohort. When compared to wildtype controls, the reduction of pro-inflammatory cytokines including Interleukin-1ß (IL1ß) and Caspase 1 contributed to the better phenotype of these mice after 24 months of age. Furthermore, these Pla2g7−/− mice revealed a shift in macrophage polarization, with fewer pro-inflammatory M1 macrophages and more immunoregulatory M2 macrophages circulating in the bloodstream. In PLA2G7-depleted macrophages, total systemic inflammation was reduced, resulting in significantly reduced Interleukin 6 (IL6) and Interleukin 12 (IL12) levels as well as tumor markers. This figure was designed using smart.servier.com