Abstract
Background
The first wave of COVID-19 pandemic may have significantly impacted antimicrobial consumption in hospitals. The objective of this study was to assess the evolution of carbapenem consumption and describe the implemented measures during the first year of the COVID-19 pandemic.
Methods
We calculated carbapenem consumption for all the hospital and for intensive care units (ICU) for three periods: baseline (before COVID-19 cases, January 2019–February 2020), and the period of COVID-19 cases as a pre-intervention (March–August 2020) and a post-intervention phase (September 2020–December 2021).
Results
During the study period, the percentage of admitted COVID-19 patients increased in the months of April–August of 2020 (pre-intervention period) from 5 to 26% of total admitted patients. The consumption of carbapenems (DDD/1000 patient days) increased from a mean of 67.1 at baseline to 142.9 pre-intervention. In ICUS, there was an increase in the mean from 125.7 to 240.8 DDD/1000 patient days. After interventions, the DDD/1000 patient days decreased by 49.5% overall the hospital and by 36% in ICUs. For the post-intervention period, there was a correlation between COVID-19 cases and carbapenem usage in the ICU but not the overall hospital.
Conclusion
An increase in the antimicrobial consumption during the first wave of COVID-19 pandemic was noticed, especially in the ICU. Antimicrobial stewardship programs are essential to reduce consumption rate.
Keywords: Antimicrobial stewardship (ASP), COVID-19, Defined daily doses (DDD), Antimicrobial resistance, Antibiotic consumption
Introduction
The emergence of the Coronavirus Disease-19 (COVID-19) pandemic had been associated with a significant impact on the utilization of antimicrobial therapy. One main reason leading to the over prescription of antimicrobials was the concern about the presence of bacterial co-infection. Such fear of bacterial co-infection may had been extrapolated from infections with influenza where bacterial co-infection was thought to be common but based on a small study of 24 patients [1]. However, in the case of the COVID-19, one study showed that only 7% of admitted patients with COVID-19 had bacterial co-infection and more than 90% received empirical antibiotics [2]. Of the common bacterial co-infections were Mycoplasma pneumoniae, Pseudomonas aeruginosa, and Haemophilus influenzae [2]. A meta-analysis of 31,953 SARS-CoV-2 patients showed a pooled proportion of laboratory-confirmed bacterial infection of 15.9% [3].
There are studies showing an increase in antimicrobial use during the first wave of the COVID-19 pandemic [4, 5]. In two studies, the percentage of patients who received antibacterial agents was 60–80% of patients with COVID-19 [6, 7]. The increased antimicrobial usage during COVID-19 may accelerate the threat of antimicrobial resistance [8]. Thus, antimicrobial agents should not be used except in the presence of high clinical suspicion of bacterial co-infection and for critically-ill patients [9]. However, not all studies described the utilization of specifically of broad-spectrum antimicrobial agents and we are not aware of such studies from Saudi Arabia although there are studies of antibiotic usage prior to the COVID-19 pandemic [10, 11]. The objective of this study was to analyze the evolution of monthly Defined Daily Dose (DDD)/1000 patients-days in three different time-periods: baseline, pre-intervention and post- intervention period.
Materials and methods
This is a retrospective study in a tertiary hospital in Eastern province, Kingdom of Saudi Arabia. The hospital has 335 beds including a 10-bed medical intensive care unit (ICU) and a 12 bed-surgical ICU. To assess the potential impact of the COVID-19 pandemic on carbapenem consumption, we included three periods: baseline (before COVID-19 cases, January 2019–February 2020), and two period with COVID-19 cases. These two periods included a pre-intervention (March–August 2020) and a post-intervention phase (September–December 2021). The period from March to August 2020 encompasses the first wave of the COVID-19 pandemic in Saudi Arabia [12–14]. The study was approved by the hospital IRB (AFHER-IRB-2022-006).
Interventions
Strengthening of the stewardship program was started after the strike increase in carbapenem consumption between March and August 2020. In addition to restriction policy for certain antimicrobials, we added frequent audit and feedback to prescribers including multidisciplinary antimicrobial rounds especially in ICUS, a 24-h on call schedule for infectious disease, a dedicated clinical pharmacist, regular monitoring, tracking and reporting of antimicrobial consumption per area and service.
Statistical analysis
Carbapenem consumption data were calculated using Defined Daily Dose (DDD)/1000 patients-days for the overall hospital and the ICUs. Using the patient-days per month for each ward, we calculated the monthly DDD/1000 patients-day. Moreover, the proportion of patients with COVID-19 was calculated by dividing the number of admitted COVID-19 cases by the total number of admissions for the hospital and ICU.
The evolution of DDDs was analyzed through multiple linear regression with time as covariate and adding a change point on September to check possible trend changes after starting the interventions. The data has been analyzed using analysis of variance (ANOVA) with the statistical package for social sciences (SPSS) version 28. All statistical test has been done with two sided. P values < 0.05 were considered statistically significant.
Results
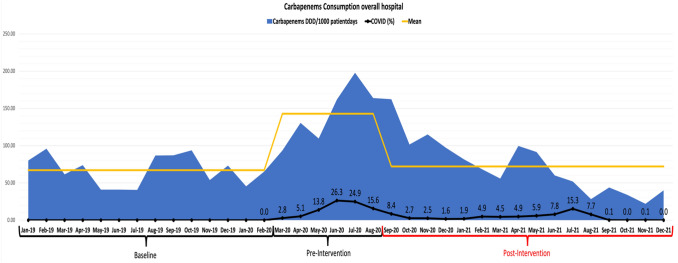
The overall hospital consumptions of carbapenems showed initial fluctuation of the DDD per 1000 patient-days during the pre-intervention phase, March-August 2020, with a mean of 67.1 ± 20.2 (Fig. 1). This was followed by an increase in the amount during the pandemic pre-intervention phase to 142.9 ± 38.8 (P value < 0.0001). The carbapenem consumptions then decreased to 72.08 ± 37.65 DDD/1000 patient-days, corresponding to a 50% reduction in the post-intervention compared to the pre-intervention period (Fig. 2).
Fig. 1.
Carbapenem consumption overall hospital during the baseline, pre-intervention, and post-intervention period
Fig. 2.
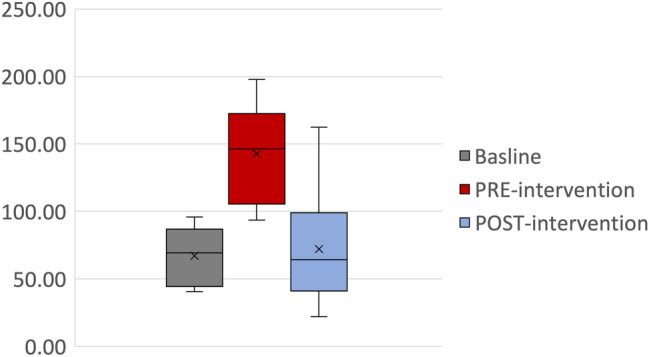
Box-and-whisker plot of Carbapenem Utilization (DDD/1000 patient-days) across the hospital during the baseline, pre-intervention, and post-intervention periods
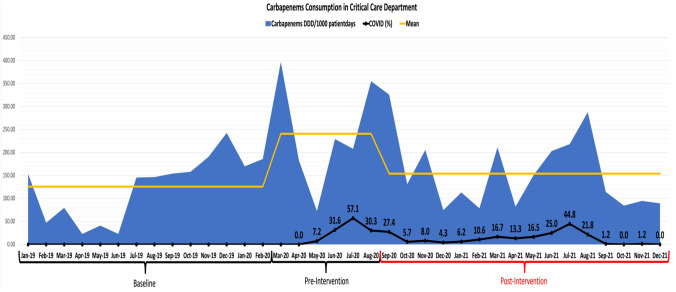
For the use of carbapenems in the ICUs, the usage increased from a mean of 125.7 DDD/1000 patient-days at baseline to 240.8 DDD/1000 patient-days pre-intervention (Fig. 3) (P value = 0.03). Following the interventions, there was a significant reduction in the usage with a mean of 154.08 ± 78.89 (P = 0.03). This reduction represented a 36% reduction in DDD/1000 patient-days (Fig. 4).
Fig. 3.
Carbapenems consumption in critical care Department during the baseline, pre-intervention, and post-intervention period
Fig. 4.
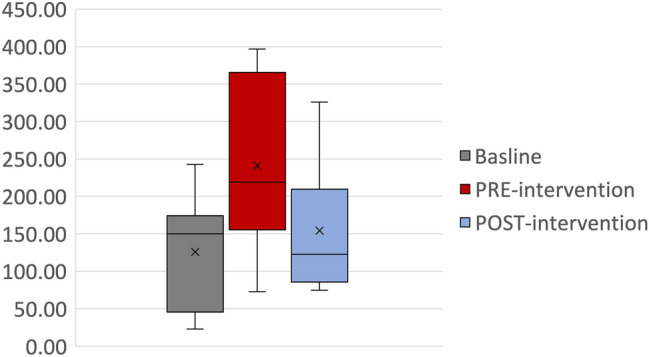
Box-and-whisker plot of Carbapenem Utilization (DDD/1000 patient-days) in intensive care units during the baseline, pre-intervention, and post-intervention periods
Correlation between percentage of admitted COVID-19 patients and carbapenem usage
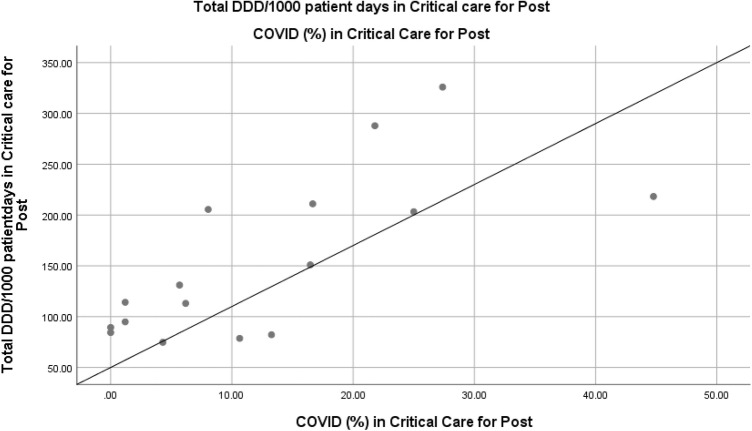
There was no statistical correlation in the pre-intervention period for all hospital data (Spearman’s Correlation = 0.77; P value = 0.07) or for the ICU (Spearman’s Correlation = − 0.03; P value = 0.96). For the post-intervention period, there was no correlation for the overall hospital (Spearman’s Correlation = 0.34; P value = 0.20) but there was a correlation for the ICU cases and carbapenem usage (Fig. 5).
Fig. 5.
Correlation between carbapenems DDD/1000 patient-days and the percentage of admitted COVID-19 patients in ICUs
Discussion
In this study, we showed increased carbapenem usage during the COVID-19 pandemic. Similarly, a previous study showed increased carbapenem usage in addition to the usage of other broad-spectrum antibacterial agents belonging to the WHO “Watch” group [15]. The use of broad-spectrum antibiotics such as carbapenems was reported from Jordan despite an overall reduction of antibiotic usage during the COVID-19 pandemic [16]. However, two other studies showed reduction in antibiotic usage in the United Kingdom during the COVID-19 pandemic [17, 18]. A systematic review showed that carbapenems were among the most commonly used antibiotics in ICUs during the COVID-19 pandemic [19]. A meta-analysis of 154 studies and 30,623 patients showed a 74.6% prevalence of antibiotic prescribing despite a low prevalence of 8.6% of bacterial co-infection [20]. In two other meta-analyses, 71.9% of patients admitted with COVID-19 received antibiotics and only 3.5% (95% confidence interval (CI), 0.4–6.7%) had bacterial co-infection [21, 22]. The clinical presentation was the most central reason for initiating antibiotics [23].
We had found a correlation between the usage of carbapenems in ICU and the percentage of admitted patients with COVID-19. Similarly, one study showed a significant usage of carbapenems, ceftriaxone, daptomycin, azithromycin, and linezolid in ICUs [24]. In another study from Brazil, antimicrobial consumption in ICU has increased during the first wave of the COVID-19 pandemic [25]. The increased use of antibiotics is related to the increased disease severity as well as the need for multiple interventions especially among ICU patients [26]. In a study from China during the COVID-19 pandemic, there was a significant increase in antimicrobial usage in hospitals with a preferential use of penicillin and cephalosporin as well as injectable agents [27]. Globally, there was an increase in the consumptions of antimicrobials in the initial phase of the COVID-19 pandemic [28]. The use of broad-spectrum antibiotics was lower during the second wave compared to the first wave as indicated by one study with reduction in the percentage of patients receiving such therapy (93.0% vs 81.7%; P < 0.01), as well as reduction in the duration of meropenem [29]. This may indicate the evolution of the experience as well as strengthening antimicrobial stewardship.
We had shown that intensifying antimicrobial stewardship resulted in a significant reduction in the used carbapenems even during the COVID-19 pandemic. Antimicrobial stewardship is based on multiple components including prospective audit and feedback. However, such activity is needed to be continuous and not intermittent in order for such activity to succeed [30]. Contributing factors to the excess use of antimicrobials during the COVID-19 pandemic include initial unfamiliarity of how to deal with SARS-CoV-2 infection; the lack of therapeutic protocols; and possible reduction in antimicrobial stewardship activity (ASP). A negative impact of the COVID-19 pandemic was described affecting many ASP activities including: audit, education, meetings, and multidisciplinary working rounds [28]. Thus, there is a need to continue aggressive ASP during the pandemic and to strengthen these efforts and provide valuable recommendations [31, 32]. An appropriate use of microbiology tests, utilizing local guidelines are important to improve antibiotic use during subsequent waves of the pandemic [31]. The use of biomarkers such as procalcitonin or C-reactive protein in this regard is not well established [33].
The present study is not exempt from limitations. A comparison of the incidences of the different infections in the compared periods would have been of interest for a more precise justification of the remarked observations or of the possible increase in the resistance rate of specific microorganisms. Unfortunately, as the study was based on antimicrobial consumption data, we did not collect information on whether the use of antibiotics was empiric or based on identified bacterial co-infections. In addition, the data was from a single center and the findings might not be applicable to other healthcare settings.
To conclude, we described an increase in the use of antimicrobials during the first wave of the COVID-19 pandemic, which was more evident in the ICU. The findings underscore the importance of having an active ASPs to optimize antimicrobial use in hospitals especially if future waves to occur.
Funding
None.
Declarations
Conflict of interest
The authors declare that they have no conflict of interests, financial or otherwise.
References
- 1.Youngs J, Wyncoll D, Hopkins P, Arnold A, Ball J, Bicanic T. Improving antibiotic stewardship in COVID-19: bacterial co-infection is less common than with influenza. J Infect. 2020;81:e55–e57. doi: 10.1016/j.jinf.2020.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alhumaid S, Al Mutair A, Al Alawi Z, Alshawi AM, Alomran SA, Almuhanna MS, et al. Coinfections with bacteria, fungi, and respiratory viruses in patients with sars-cov-2: a systematic review and meta-analysis. Pathogens. 2021 doi: 10.3390/pathogens10070809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu NJ, McLeod M, McNulty CAM, Lecky DM, Holmes AH, Ahmad R. Trends in antibiotic prescribing in out-of-hours primary care in england from January 2016 to June 2020 to understand behaviours during the firstwave of COVID-19. Antibiotics. 2021;10:1–10. doi: 10.3390/antibiotics10010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guisado-Gil AB, Infante-Domínguez C, Peñalva G, Praena J, Roca C, Navarro-Amuedo MD, et al. Impact of the COVID-19 pandemic on antimicrobial consumption and hospital-acquired candidemia and multidrug-resistant bloodstream infections. Antibiot (Basel, Switzerland) 2020;9:1–11. doi: 10.3390/ANTIBIOTICS9110816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothe K, Feihl S, Schneider J, Wallnöfer F, Wurst M, Lukas M, et al. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: a retrospective cohort study in light of antibiotic stewardship. Eur J Clin Microbiol Infect Dis. 2021;40:859–869. doi: 10.1007/s10096-020-04063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karami Z, Knoop BT, Dofferhoff ASM, Blaauw MJT, Janssen NA, van Apeldoorn M, et al. Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: results from a multicentre retrospective cohort study in The Netherlands. Infect Dis (Auckl) 2021;53:102–110. doi: 10.1080/23744235.2020.1839672. [DOI] [PubMed] [Google Scholar]
- 8.Hsu J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ. 2020 doi: 10.1136/bmj.m1983. [DOI] [PubMed] [Google Scholar]
- 9.Ginsburg AS, Klugman KP. COVID-19 pneumonia and the appropriate use of antibiotics. Lancet Glob Heal. 2020;8:e1453–e1454. doi: 10.1016/S2214-109X(20)30444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Tawfiq JA. Changes in the pattern of hospital intravenous antimicrobial use in Saudi Arabia, 2006–2008. Ann Saudi Med. 2012;32:517–520. doi: 10.5144/0256-4947.2012.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Tawfiq JA, Al-Homoud AH. Pattern of systemic antibiotic use among hospitalized patients in a general hospital in Saudi Arabia. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101605. [DOI] [PubMed] [Google Scholar]
- 12.AlJishi JM, Alhajjaj AH, Alkhabbaz FL, AlAbduljabar TH, Alsaif A, Alsaif H, et al. Clinical characteristics of asymptomatic and symptomatic COVID-19 patients in the Eastern Province of Saudi Arabia. J Infect Public Health. 2021;14:6–11. doi: 10.1016/j.jiph.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AlBahrani S, Al-Tawfiq JA, Jebakumar AZ, Alghamdi M, Zakary N, Seria M, et al. Clinical features and outcome of low and high corticosteroids in admitted COVID-19 patients. J Epidemiol Glob Health. 2021;11:316–319. doi: 10.2991/jegh.k.210521.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barry M, Alotaibi M, Almohaya A, Aldrees A, AlHijji A, Althabit N, et al. Factors associated with poor outcomes among hospitalized patients with COVID-19: experience from a MERS-CoV referral hospital. J Infect Public Health. 2021;14:1658–1665. doi: 10.1016/j.jiph.2021.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castro-Lopes A, Correia S, Leal C, Resende I, Soares P, Azevedo A, et al. Increase of antimicrobial consumption in a tertiary care hospital during the first phase of the covid-19 pandemic. Antibiotics. 2021 doi: 10.3390/antibiotics10070778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Azzam S, Mhaidat NM, Banat HA, Alfaour M, Ahmad DS, Muller A, et al. An assessment of the impact of coronavirus disease (Covid-19) pandemic on national antimicrobial consumption in jordan. Antibiotics. 2021 doi: 10.3390/antibiotics10060690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezel-Potts E, L’Esperance V, Gulliford MC. Antimicrobial stewardship in the UK during the COVID-19 pandemic: a population-based cohort study and interrupted time-series analysis. Br J Gen Pract. 2021;71:E331–E338. doi: 10.3399/BJGP.2020.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu N, Aylin P, Rawson T, Gilchrist M, Majeed A, Holmes A. Investigating the impact of COVID-19 on primary care antibiotic prescribing in North West London across two epidemic waves. Clin Microbiol Infect. 2021;27:762–768. doi: 10.1016/j.cmi.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan S, Hasan SS, Bond SE, Conway BR, Aldayeb MA. Antimicrobial consumption in patients with COVID-19: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. 2021 doi: 10.1080/14787210.2022.2011719. [DOI] [PubMed] [Google Scholar]
- 20.Langford BJ, So M, Raybardhan S, Leung V, Soucy JPR, Westwood D, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27:520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langford BJ, So M, Raybardhan S, Leung V, Westwood D, MacFadden DR, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beovic B, Dousak M, Ferreira-Coimbra J, Nadrah K, Rubulotta F, Belliato M, et al. Antibiotic use in patients with COVID-19: A “snapshot” Infectious Diseases International Research Initiative (ID-IRI) survey. J Antimicrob Chemother. 2020;75:3386–3390. doi: 10.1093/jac/dkaa326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grau S, Echeverria-Esnal D, Gómez-Zorrilla S, Navarrete-Rouco ME, Masclans JR, Espona M, et al. Evolution of antimicrobial consumption during the first wave of covid-19 pandemic. Antibiotics. 2021;10:1–10. doi: 10.3390/antibiotics10020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva ARO, Salgado DR, Lopes LPN, Castanheira D, Emmerick ICM, Lima EC. Increased use of antibiotics in the intensive care unit during coronavirus disease (COVID-19) pandemic in a Brazilian hospital. Front Pharmacol. 2021 doi: 10.3389/fphar.2021.778386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Silva RMR, de Mendonça SCB, Leão IN, dos Santos QN, Batista AM, Melo MS, et al. Use of monitoring indicators in hospital management of antimicrobials. BMC Infect Dis. 2021 doi: 10.1186/s12879-021-06542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Geng X, Liu X, Wen X, Wu R, Cui D, et al. Antibiotic use in China’s public healthcare institutions during the COVID-19 pandemic: an analysis of nationwide procurement data, 2018–2020. Front Pharmacol. 2022 doi: 10.3389/fphar.2022.813213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khouja T, Mitsantisuk K, Tadrous M, Suda KJ. Global consumption of antimicrobials: impact of the WHO Global Action Plan on Antimicrobial Resistance and 2019 coronavirus pandemic (COVID-19) J Antimicrob Chemother. 2022 doi: 10.1093/jac/dkac028. [DOI] [PubMed] [Google Scholar]
- 29.Chan XHS, O’Connor CJ, Martyn E, Clegg AJ, Choy BJK, Soares AL, et al. Comparison of antibiotic use between the first two waves of COVID-19 in an Intensive Care Unit at a London Tertiary Centre: reducing broad-spectrum antimicrobial use did not adversely affect mortality. J Hosp Infect. 2022 doi: 10.1016/j.jhin.2022.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Tawfiq JA, Al-Homoud AH. Intermittent daily de-escalation rounds did not have significant impact on antimicrobial stewardship program targeting carbapenems. Int J Clin Pract. 2021 doi: 10.1111/ijcp.14507. [DOI] [PubMed] [Google Scholar]
- 31.Khor WP, Olaoye O, D’arcy N, Krockow EM, Elshenawy RA, Rutter V, et al. The need for ongoing antimicrobial stewardship during the COVID-19 pandemic and actionable recommendations. Antibiotics. 2020;9:1–12. doi: 10.3390/antibiotics9120904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierce J, Stevens MP. COVID-19 and antimicrobial stewardship: lessons learned, best practices, and future implications. Int J Infect Dis. 2021;113:103–108. doi: 10.1016/j.ijid.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulia MS, Wolf I, Schwei RJ, Chen D, Lepak AJ, Schulz LT, et al. Antibiotic prescribing patterns for coronavirus disease 2019 (COVID-19) in two emergency departments with rapid procalcitonin. Infect Control Hosp Epidemiol. 2021;42:359–361. doi: 10.1017/ice.2020.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]