Abstract
Objective:
To determine the influence of maternal trauma and posttraumatic stress disorder (PTSD) symptoms on children’s physiological response to threat and safety signals during a fear conditioning task in trauma-exposed mothers and children.
Method:
Participants were African American mother-child dyads (N = 137; children aged 8–13 years). Mothers’ trauma history and PTSD symptoms were assessed; Latent Class Analysis (LCA) was conducted from these measures to identify distinct classes. Children reported violence exposure and completed a differential fear conditioning task using fear-potentiated startle (FPS) responses to conditioned danger (CS+) and safety (CS-) signals.
Results:
Four classes of maternal trauma history and PTSD symptoms emerged: 1) Lower Trauma, 2) Moderate Trauma, 3) High Sexual Abuse, and 4) High Trauma and PTSD Symptoms. Children’s FPS to CS + and CS- were tested with maternal class as the between-subjects factor. FPS to the danger signal was not significantly different across maternal classes, but FPS to safety (CS-) was significantly higher for the Lower Trauma and High Trauma and PTSD Symptoms classes than either the Moderate Trauma or the High Sexual Abuse classes.
Conclusions:
Results indicate that maternal trauma impacts children’s ability to modulate fear responses in the presence of a safety signal, independent of the children’s own trauma exposure. To our knowledge, this is the first study to demonstrate that children’s fear inhibition is impacted by maternal trauma exposure. Prior studies have linked fear inhibition to mental health outcomes, highlighting the need to understand intergenerational modulation of fear learning and physiology.
Keywords: Fear conditioning, Startle response, Intergenerational transmission of trauma, Psychopathology, Development
1. Introduction
Trauma exposure is pervasive, with over 70 % of people globally exposed to at least one traumatic event in their lifetimes and an average of >3 trauma exposures per person [1]. These exposures have wide-ranging effects and are linked to neural [2,3], behavioral [4,5], epigenetic [6,7], physiological [8,9] and mental health effects [1,10] in trauma-exposed individuals. The timing of trauma exposure is associated with long term risk, such that childhood trauma has particularly pervasive effects on well-being [11], including compromised immune function [12,13], obesity [14], drug use [15], dysregulated stress responsivity [16], accelerated biological aging [17], and psychopathology [18,19]. A growing body of evidence now indicates trauma may also have intergenerational effects, such that parents’ trauma exposure can impact their offspring [20–24]. Although a substantial body of evidence suggests a link between parental trauma exposure and adverse child outcomes [24–27], the relevant mechanisms of transmission are an area of active investigation. These mechanisms are likely multifactorial and include epigenetic [6,28], neuroendocrine [29,30], and behavioral/environmental pathways [23,31,32].
To date, most studies of intergenerational transmission of trauma in humans have focused on how parents’ trauma exposure impacts their children’s health outcomes, such as psychopathology [28,33] and mortality [34]. Many studies have examined behavioral transmission of trauma from mothers to their children via its effects on parenting [24, 31,32]. Somewhat fewer studies have examined biological pathways through which trauma exerts intergenerational effects in humans (for reviews see [35,36]), and only a handful have examined the relation between trauma exposure in one generation and intermediate risk phenotypes in subsequent generations [8,37]. Animal studies have allowed for more systematic investigation of how exposure to traumatic stressors in one generation impacts subsequent generations through biological pathways that modulate risk phenotypes (for reviews see, e.g., Chan, Nugent, & Bale, 2018; Cowan, Callaghan, Kan, & Richardson, 2016). These studies have identified intergenerational effects of stress on offspring’s stress responsivity [38,39] caregiving [33,40], and fear learning [41]. In one study, male rats (F0) completed an odor conditioning task prior to reproduction [41]. Two generations (F1 and F2) of their subsequently conceived offspring were then tested with the same odors pairings; both generations of offspring displayed behavioral sensitivity to the fear-conditioned odor in the F0 rats. This intergenerational sensitivity persisted even when the offspring were conceived via in-vitro fertilization and cross-fostered, consistent with the hypothesis that the father’s learned fear had been transmitted via germ line cells to the offspring. This finding raises the possibility that parental exposure to threats could directly impact their offspring via biological transmission of fear responsivity.
Fear learning, or conditioning, is a behavioral measure that has been associated with risk for psychopathology [37,42,43]. This makes it an ideal paradigm for use in further elucidating how parental trauma exposure impacts their children. Fear conditioning is a type of Pavlovian conditioning model wherein a neutral conditioned stimulus (CS; e.g., a shape) is paired with an aversive unconditioned stimulus (US; e.g., a shock). After the CS and US are paired repeatedly, an association is formed such that the CS alone elicits the conditioned response (e.g., a fear response). Two physiological responses have been used as behavioral outcome measures for fear conditioning in humans: acoustic startle response and skin conductance response (SCR). The acoustic startle response is characterized by an integrative, reflex contraction of the skeletal musculature in response to a sudden intense environmental stimulus [44]. It is mediated by a simple subcortical three-neuron circuit [45], but is modulated by brain structures including the amygdala [46] and prefrontal cortex, which are implicated in anxiety-, trauma-, and stress-related disorders [37]. Fear-potentiated startle (FPS) is the relative increase in the startle response elicited in the presence of a conditioned stimulus that was previously paired with an aversive stimulus [45].
A handful of prior studies have examined the effects of trauma per se on physiological indices of startle response. One reported that childhood sexual and physical abuse were associated with increased acoustic startle eye blink response magnitude, and that the association was not explained by current psychopathology [47]. A study of children with and without maltreatment exposure found that the maltreated children had blunted skin-conductance responses during conditioning and differentiated between threat and safety signals less effectively, relative to the non-maltreated children [48]. This disrupted response pattern mediated the association between maltreatment and externalizing psychopathology. To our knowledge, however, two studies have examined intergenerational effects on physiological responses to threat and safety cues [49][50]. One examined the impact of major depressive disorder (MDD) on startle response across three generations [49]. The parental generation included individuals with and without MDD. Their children and grandchildren completed a fear conditioning task while acoustic startle responses were measured. Children of the MDD participants had larger startle responses than the non-MDD’s children. Elevated startle was also apparent in the MDD’s female, but not male, grandchildren. Another study reported that children of depressed mothers had larger SCRs to the CS- than to the CS + during acquisition, and lower SCRs to CS + compared to control or anxious mothers [50]. These results suggest that maternal depression may be associated with blunted SCRs to threat cues, however, this study did not examine maternal trauma history as a potential factor in offspring fear learning. Although these studies did not assess trauma, this pattern of results indicates that fear learning may be a marker of intergenerational risk for psychopathology.
The prospect of intergenerational effects on fear response and learning suggests an important risk factor for psychopathology, as these processes have been implicated in the pathophysiology of fear and anxiety disorders [8,37,51]. A seminal finding from fear conditioning studies is that elevated fear response to safety signals is a biomarker of PTSD [52,53] and has been implicated in anxiety disorders generally [43]. Individuals with PTSD show similar fear acquisition relative to non-PTSD controls, but they are less able to discriminate between threat and safety cues [52,53]. Developmental studies have reported that elevated response to safety signals in the context of a fear learning task is associated with anxiety symptoms in middle childhood and early adolescence [55]. Together, these findings suggest that fear learning, and particularly deficient fear inhibition in the presence of a safety signal, may be an intermediate risk phenotype that partially mediates the relationship between parental trauma and their offspring’s increased risk for psychopathology.
To our knowledge there have not yet been direct tests for transmission of specific learned fear in humans, and few papers have tested for associations between parental risk factors (trauma or psychopathology) and fear learning in subsequent generations [26,49,50]. However, multiple studies indicate that maternal trauma exposure is linked to altered responsivity of the hypothalamic-pituitary-adrenal (HPA) axis in offspring [25,29,56,57], and, in turn, HPA axis function has been linked to modulation of the acoustic startle response and fear learning [58,59]. There is increasing evidence that parental trauma exposure modulates methylation of genes involved in regulation of the HPA axis [28,60], and studies have linked maternal trauma exposure to lower cortisol levels in their infant children [21,29]. These findings indicate that a key neuroendocrine system is impacted by intergenerational trauma and suggest that one route through which maternal trauma exposure impacts fear learning in children is via effects of the HPA axis.
The objective of the present study was to evaluate whether maternal trauma history and symptoms were related to children’s fear-potentiated startle responses to threat or safety cues during a fear acquisition task. Importantly, the study participants were all African American mothers and children with generally high levels of trauma exposure [26,61]. Evidence suggests that the type and timing of trauma exposure are related to later outcomes, therefore, we used a data-driven approach to identify subgroups of mothers based on their prior traumatic experiences and current PTSD symptoms. We then compared the children’s startle responses to threat and safety cues between each maternal subgroup. Based on the prior research indicating both effects of maternal trauma on the HPA axis and the role of the HPA axis in modulating fear learning and response, we hypothesized that the extent of maternal trauma exposure would impact their children’s ability to inhibit fear in the presence of the CS-.
2. Methods
2.1. Participants
Participants were 321 mothers and 137 mother-child dyads recruited from a larger study of African American primary caregivers and children from a low-income, urban population with high trauma exposure [26, 62]. To assess whether maternal trauma history and symptoms were associated with their children’s physiologic response to a fear acquisition task, only dyads with complete maternal trauma history and children acquisition data were included in the final sample, N = 137. All mothers were their child’s legal guardian and primary caretaker. The children (74 girls) were 8 to 13-years-old, M = 10.2, SD = 1.4. The mothers were 21- to 59-years-old, M = 35.3, SD = 8.3. 82.4 % reported an average monthly household income <$2,000, and 50.7 % reported having either high school or less formal education. Their trauma exposure ranged from 0 to 12 distinct Criterion A traumas according to the Diagnostic and Statistical Manual of Mental Disorders (DSM–5) and 92.6 % reported experiencing at least one trauma. Participants were recruited from the waiting rooms of the Primary Care or Obstetrics Gynecology clinics at the Grady Health System in Atlanta, GA. Exclusion criteria included autism spectrum disorders, bipolar or psychotic disorders, and cognitive disability; these were determined by a History and Physical examination by the study physician. Prior to participation, all mothers signed informed consent as well as parental permission for their children, and the children provided study assent approved by the Emory University Institutional Review Board and the Grady Research Oversight Committee.
2.2. Clinical assessments
Mother’s child abuse exposure was assessed using the Childhood Trauma Questionnaire (CTQ) [63]. The CTQ is a 28-item psychometrically validated, self-report inventory assessing self-reported level of child abuse and neglect (Bernstein et al. [64]). The CTQ yields scores for five types of abuse and neglect: sexual abuse, physical abuse, physical neglect, emotional abuse, and emotional neglect. The total score sums across all five subscales.
Mother’s adult trauma exposure was assessed via the Traumatic Events Inventory (TEI), which assesses lifetime history of trauma exposure [65]. The TEI assesses past experience and frequency of 13 separate types of Criterion A traumatic events. To summarize level of exposure to trauma other than child abuse, we summed total number of different types of non–child abuse trauma exposure reported by each participant.
Mothers who endorsed at least one Criterion A trauma had PTSD symptoms assessed relative to their self-reported worst trauma, using the modified PTSD Symptom Scale (PSS; [66]), a psychometrically valid 17-item self-report measure of current symptoms of PTSD based on DSM-IV criteria, with scores ranging from 0 to 47. The PSS has been validated with the widely used measure of PTSD: the Clinician Administered PTSD Scale [66], and was only completed on mothers reporting exposure to Criterion A trauma. The PSS frequency items were summed to obtain a continuous measure of PTSD symptom severity.
Children’s exposure to violence was assessed with the Violence Exposure Scale for Children-Revised (VEX-R; Fox & Leavitt, 1995). This 25-item scale comes in male and female versions. Internal consistency ranges from 0.80 to 0.86. The VEX-R was correlated with parental report of child trauma exposure on the Trauma Exposure Screening Inventory (TESI; Ghosh-Ippen et al., 2002), r(149) = .20, p = .017.
2.3. Psychophysiological assessment
The electromyogram (EMG) data were acquired at a sampling rate of 1000 Hz using Biopac MP150 for Windows (Biopac Systems, Inc., Aero Camino, CA). The data were filtered, rectified, and smoothed in MindWare software (MindWare Technologies, Inc.) and exported for statistical analyses. EMG activity was recorded from two 5 mm Ag/AgCl electrodes placed over the orbicularis oculi muscle, approximately 1 cm under the pupil and 1 cm below the lateral canthus. The impedances for all participants were less than 6kΩ. The EMG signal was filtered with low- and high-frequency cutoffs at 28 and 500 Hz, respectively. Startle magnitude was assessed as the peak amplitude of the EMG contraction 20–200 ms following the acoustic startle probe, which was a 106-dB [A] SPL, 40-ms burst of broadband noise delivered binaurally through headphones.
2.4. Experimental design
The experimental paradigm assessed differential fear conditioning, based on a paradigm used successfully in both child and adult trauma populations [55,67]. See Fig. 1. Participants were seated in a sound attenuated booth and asked to remain still and watch the computer monitor, as one shape was followed by an airblast and the other shape was not. The fear conditioning protocol consisted of a habituation period, which included two trials of each type: noise alone (NA) and two CSs, one of which was reinforced during acquisition. The NA trials were included as a baseline response, in order to measure individual variability in acoustic startle response. The acquisition phase followed habituation and contained 3 blocks. Each block included 3 CS+, 3 CS-, and 3 NA trials; there were 27 startle trials total. The unconditioned stimulus (US) was an aversive airblast to the larynx at 80 pounds per square inch. Both CSs were colored shapes presented on a computer monitor using Superlab presentation software (Cedrus, Inc.) for 6000 ms prior to the startle probe. The CS + co-terminated with the US 500 ms after the presentation of the startle stimulus. The CS + was reinforced with the airblast on 100 % of the trials.
Fig. 1.
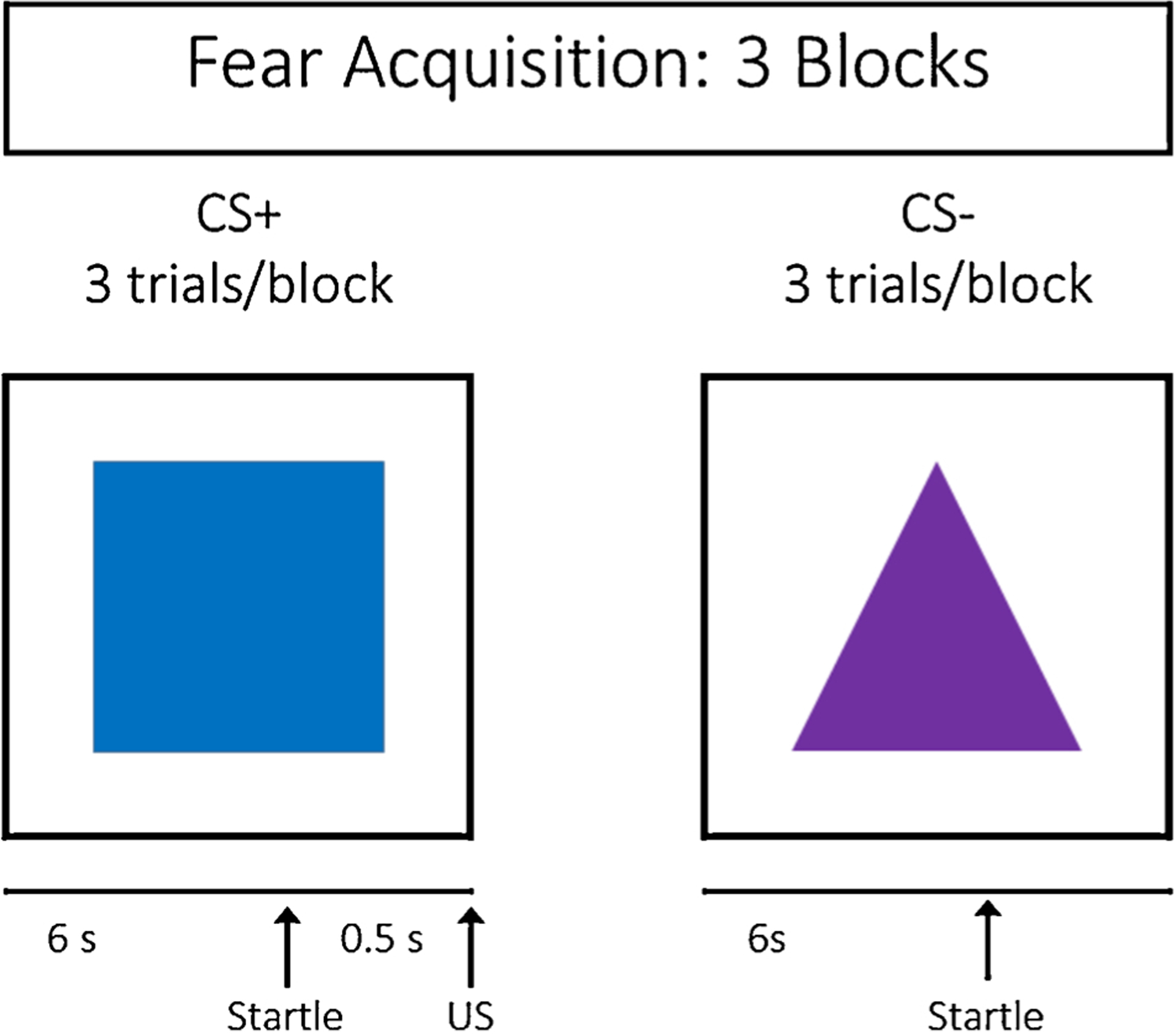
Fear acquisition paradigm.
2.5. Analytic approach
All analyses were conducted in SPSS version 26 and Mplus version 8.4. Statistical significance was evaluated using p-values and α = .05 threshold for parametric tests. The significance of non-parametric tests was determined using 95 % confidence intervals (CIs), such that CIs that did not include 0 indicate significance. Tukey’s Honestly Significant Difference test was used for all post-hoc comparisons.
We used Mplus to generate empirically based classes of mothers based on their trauma history and post-traumatic stress disorder symptoms using Latent Class Analysis (LCA). LCA is a statistical method that identifies heterogeneity in a population and then identifies subgroups, or classes, that have similar features [68]. LCA assumes there are discrete latent classes of participants with similar probabilities of endorsing a common set of responses, such as trauma exposures and symptoms [69]. This approach elucidates subgroups of participants based on similar responses, and then evaluates whether these N subgroups (e.g., 3) characterize the sample more accurately than N −1 subgroups (e.g., 2). We assessed model fit in two ways. First, we compared Bayesian Information Criteria (BIC) across models (i.e., 2 versus 3 classes, 3 versus 4 classes); models with lower BICs indicate a better fit than those with higher values. Second, results from the Vuong-Lo-Mendell-Rubin (VLMR) likelihood ratio test indicated whether the model with N classes was a significantly better fit than the a model with N-1 classes [68]. All total scores for adult trauma, childhood trauma, and current PTSD symptoms were z-scored prior to conducting the LCA.
Children’s startle data was evaluated for learning effects with a repeated-measures Analysis of Variance (ANOVA) with the factors Trial Type (NA, CS+, CS-) and Block (Habituation, 1, 2, 3). Sphericity was evaluated with Mauchly’s Test and Greenhouse-Geisser corrections were applied in case of violations. Subsequent analyses were stratified by CS Type given that we hypothesized effects of maternal trauma on child FPS could be specific to CS-. As in our prior studies, FPS was calculated as a difference score between CS and NA to capture effects of learning above the individual’s baseline acoustic startle response [55,70–72]. We also focused class analyses on late acquisition, defined as averaged FPS during Blocks 2 and 3 in order to capture maximal learning [55,70–72]. We assessed the impact of mother’s latent class membership on their child’s fear and safety learning across acquisition with univariate ANOVAs. Because the class sizes differed substantially, results of these ANOVAs were based on 5000 bootstrapped resamples of the data. Bootstrapping is a non-parametric statistical method that resamples with replacement from the sample to generate to a null distribution that reflects characteristics of the population [73–75]. This non-parametric approach does not assume any characteristics of the true population distribution (e.g., normality). Test statistics and confidence intervals are then generated from this null distribution.
3. Results
3.1. Latent class analysis
Out of the 321 mothers enrolled, 209 (65 %) were categorized as Class 1, the Lower Trauma class, 32 (10 %) were categorized as Class 2, the Moderate Trauma class, 48 (15 %) were categorized as Class 3, the High Childhood Sexual Abuse class, and 32 (10 %) were categorized as Class 4, the High Trauma and PTSD Symptoms class. Descriptive statistics for key variables are reported by class in Table 1. Maternal age did not differ significantly between classes, F(3,134) = 1.60, p = .19, but child abuse, trauma, and PTSD symptoms did, all ps < .001, see Table 1.
Table 1.
Mother’s child abuse, adult trauma, and PTSD symptoms, and children’s age, violence exposure (VEXR), and trauma exposure (TESI), by maternal Class assignment. Means followed by standard deviations, M(SD), and results of a one-way analysis of variance that tested for class differences.
| Lower Trauma | Moderate Trauma | High Sex Abuse | High Trauma & PTSD Symptoms | F | |
|---|---|---|---|---|---|
| Mother | |||||
| Age | 35.2 (8.4) | 31.0 (5.5) | 37.4 (8.9) | 36.1 (8.2) | N.S. |
| Child abuse | 31.3 (7.2) | 62.9 (9.1) | 61.8 (10.0) | 91.9 (9.0) | (3,136) = 283.8** |
| Adult traumas | 3.5 (2.4) | 5.8 (2.0) | 5.2 (2.6) | 8.3 (2.8) | (3,134) = 14.7** |
| PTSS symptoms | 10.6 (10.5) | 18.7 (10.1) | 22.2 (12.5) | 30.4 (11.3) | (3,132) = 20.0** |
| Child | |||||
| Age | 10.0 (1.6) | 10.0 (1.9) | 10.6 (1.5) | 10.1 (1.3) | N.S. |
| Violence (VEX-R) | 8.2 (4.0) | 8.2 (4.3) | 8.9 (4.7) | 10.3 (5.1) | N.S. |
| Trauma (TESI) | 4.5 (2.7) | 4.7 (3.1) | 4.9 (2.2) | 6.0 (3.6) | N.S. |
Note. N.S. indicates a non-significant F-test.
indicates p < .001.
3.1.1. Lower trauma class
Post-hoc class comparisons indicated that the Lower Trauma class had significantly lower levels of child abuse, adult trauma exposure, and PTSD symptoms, relative to the other classes, all ps < .02, except for non-significant differences in sexual abuse from the Moderate Trauma class, p = .99, and in physical neglect from the High Sexual Abuse class, p = .28.
3.1.2. Moderate trauma class
The Moderate Trauma class reported less sexual abuse than the High Sexual Abuse class, p < .001, more physical abuse than either the Lower Trauma or High Sexual Abuse class, both ps < .03, less physical abuse than the High Trauma and PTSD Symptoms class, p < .001, more adult trauma than the Lower Trauma class, p = .02, and lower PTSD symptoms than the High Trauma and PTSD Symptoms class, p = .002.
3.1.3. High sexual abuse class
The High Sexual Abuse class reported higher childhood sexual abuse than either the Lower Trauma or Moderate Trauma classes, both ps < .001. The High Sexual Abuse class had significantly higher childhood physical abuse, emotional abuse, physical neglect, emotional neglect, adult trauma, and PTSD symptoms than the Lower Trauma class, all ps < .01. In contrast, the High Sexual Abuse class reported less physical abuse, physical neglect, and emotional abuse than the Moderate Trauma class, all ps < .03. The High Sexual Abuse class reported less physical abuse, emotional abuse, emotional neglect, physical neglect, and adult trauma than the High Trauma and PTSD Symptoms class, all ps < .01.
3.1.4. High trauma and PTSD symptoms class
The High Trauma and PTSD Symptoms class reported higher physical abuse, physical neglect, and emotional neglect than the other three classes, all ps < .03. It also had higher emotional abuse and adult trauma exposure than Lower Trauma and High Sexual Abuse groups, all ps < .01. The High Trauma and PTSD Symptoms class had higher levels of PTSD symptoms than the Lower Trauma and Moderate Trauma classes, both ps < .01.
Classification specificity was high: all classes had > 93 % probability of being assigned to the class there were in, and < 7% chance of being assigned to any other class. The BIC was 6014.9 for the 4-class model versus 6314.1 for the 2-class model and 6144.3 for the 3-class model, indicating that the 4-class model was more parsimonious. The VLMR test also indicated that the 4 classes improved the model fit versus a 3-class model, p = .005. The entropy of the 4-class model (0.964) surpassed the threshold for good class separation (i.e., entropy = 0.60; Asparouhov & Muthén [76]), and exceeded that of the 2- and 3-class models, with entropy values of 0.96 and 0.92, respectively. See Table 1 and Fig. 2.
Fig. 2.
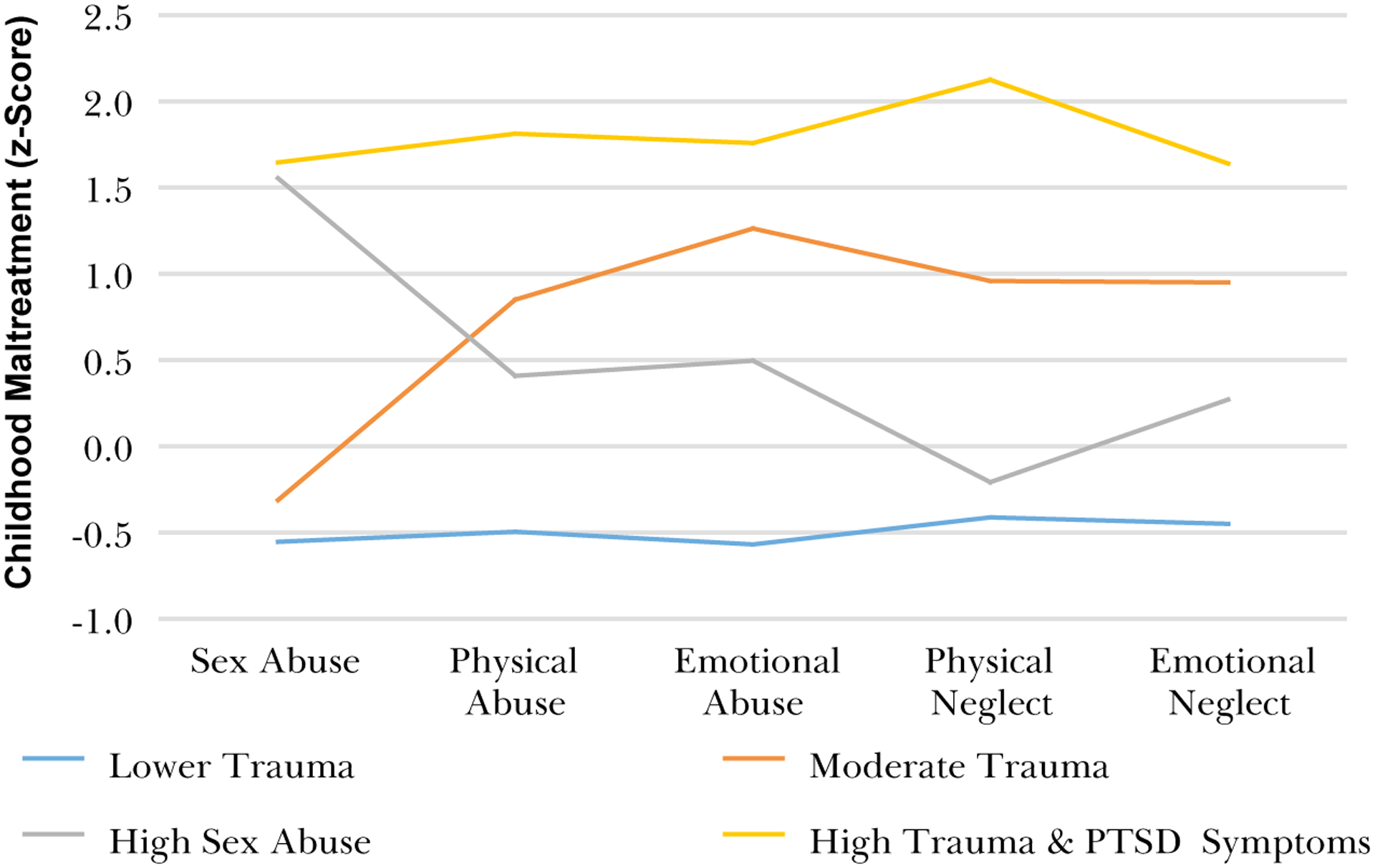
Relative exposure (z-scores) to five types of child abuse within each Class of mothers.
3.2. Child startle data
We conducted a repeated-measures Analysis of Variance with the factors Block (Habituation, 1, 2, and 3) and Trial Type (CS+, CS-, and NA) to assess fear learning across the acquisition task. There were significant main effects of Trial Type, F(2,298) = 13.1, p < .001, ɳ2p = .08, and Block, F(3,447) = 12.98, p < .001, ɳ2p = .08, indicating that startle magnitude differed as a function of Trial Type and Block. The Trial Type by Block interaction was significant, F(6,894) = 5.64, p < .001, ɳ2p = .04, indicating change in response to each Trial Type across Blocks. Results of within-subjects contrasts indicated significant differences between all Trial Types, all ps < .005, and between responses during Habituation versus Block 1, p = .005, and between Blocks 1 and 2, p = .003, but not between Blocks 2 and 3, p = .26. The Trial Type by Block interactions was driven by significant change between Blocks 1 and 2 in startle to NA versus CS+, p = .03, and CS+ versus CS-, p = .02. See Fig. 3. We focused on late acquisition in subsequent analyses.
Fig. 3.
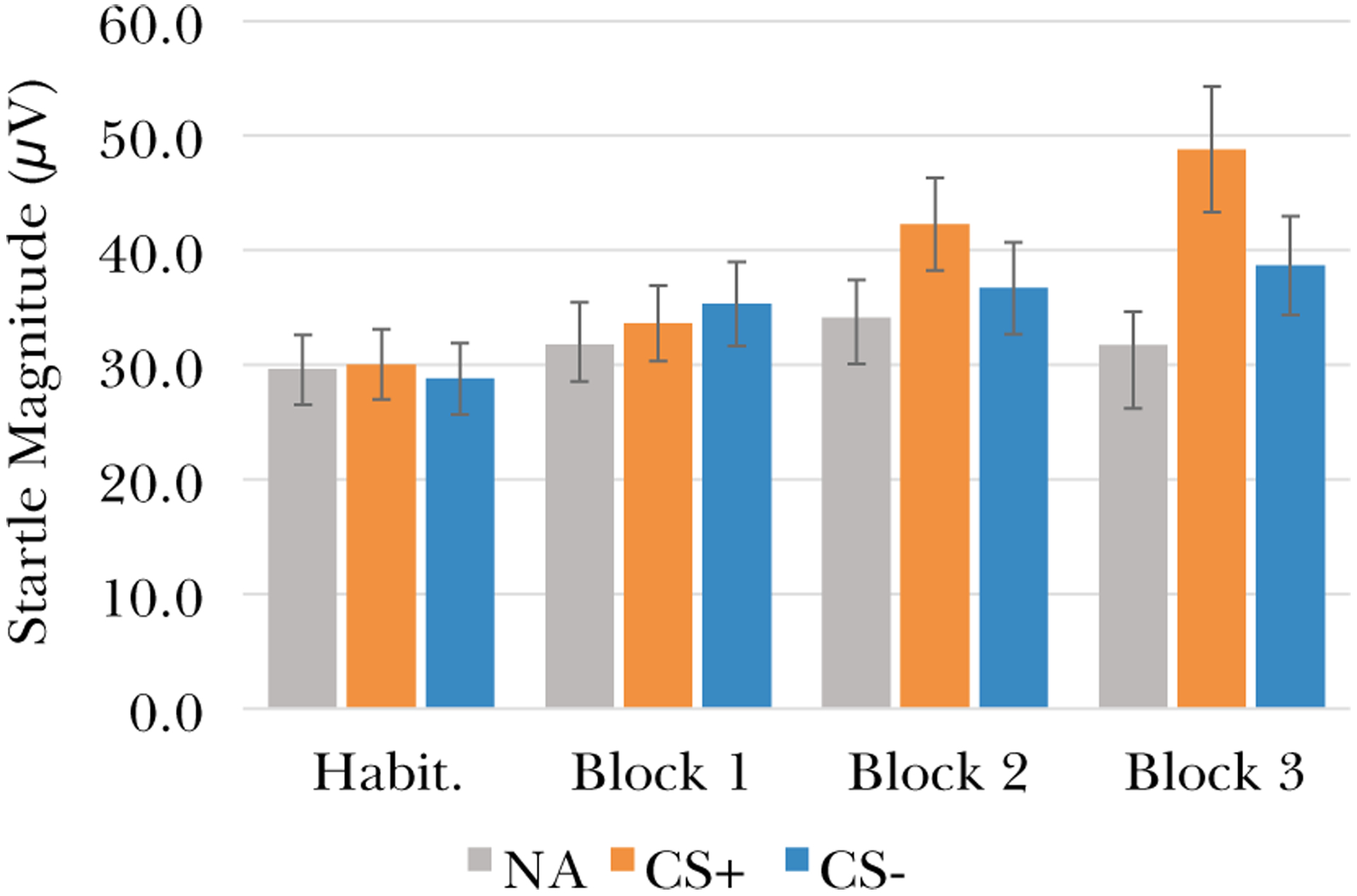
Eyeblink startle magnitude for each trial type across the fear acquisition task indicates that fear responses to the CS + increased across the task relative to CS- and NA.
There were 137 children with startle data available; 83 had Lower Trauma mothers, 13 had Moderate Trauma mothers, 27 had High Childhood Sexual Abuse mothers, and 13 had High Trauma and PTSD Symptoms mothers. We evaluated whether threat (CS+) or safety signal (CS-) FPS during Blocks 2 and 3 differed as a function of maternal class with a 2 × 4 mixed ANOVA with the repeated measure Trial Type (2; CS + and CS-) and the between-subjects factor maternal class (4; Lower Trauma, Moderate Trauma, High Childhood Sexual Abuse, and High Childhood Sexual Abuse). The dependent variable was FPS, calculated as a difference score from NA startle. There was a significant difference between CS types, F(1,131) = 10.2, p = .002, ɳ2p = .072, as FPS was higher for the CS+, M = 8.14 μV (SD = 2.20), than the CS-, M = 0.32 μV (SD = 1.41). FPS also differed significantly as a function of maternal class, F(3,131) = 3.24, p = .024, ɳ2p = .069. Post-hoc tests indicated that this main effect of class was driven by a significantly higher startle response from the maternal Lower Trauma class than the maternal High Sexual Abuse class, MDiff = 8.32 μV, p = .015. The interaction between CS type and maternal class was not significant, F(3,131) = 1.67, p = .18, ɳ2p = .037, however, the main effects of both CS type and maternal class, as well as the class differences depicted in Fig. 4, indicated that stratification by CS type in order to permit a non-parametric examination of class effects on CS- FPS was appropriate.
Fig. 4.
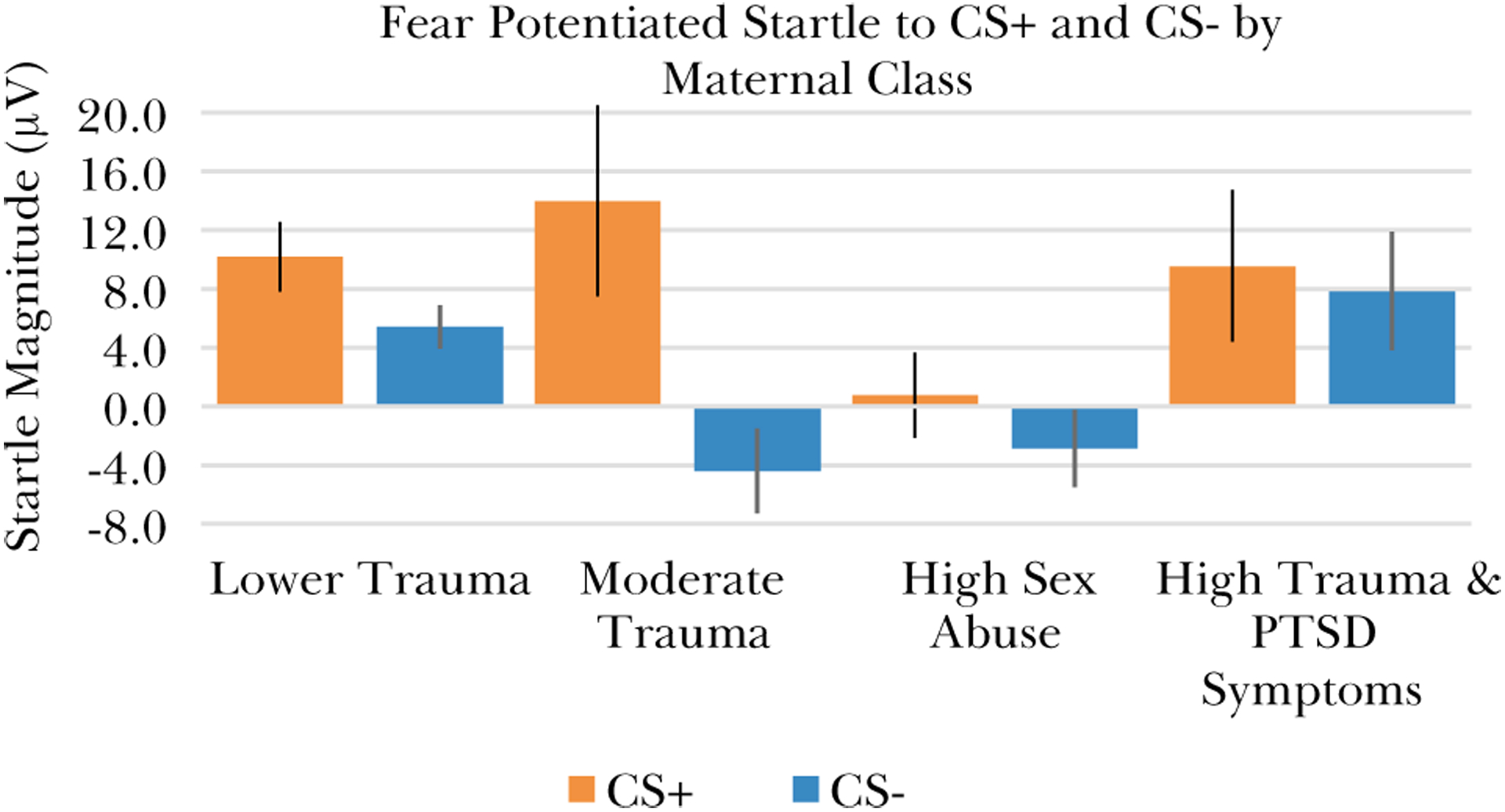
Children’s startle to the fear (CS+) and safety (CS-) signals during late acquisition (Blocks 2 and 3) differed according to their mother’s class membership. Error bars show +/− 1 standard error.
We compared FPS to the CS + and CS-, respectively, between classes in separate one-way ANOVAs that included the children’s age and exposure to violence as covariates. The ANOVA for CS + indicated there was not a significant difference in startle between classes, F(3,126) = 1.74, p =.16. In contrast, there was a significant class difference in startle to the CS-, F(5,125) = 3.25, p = .024. Post-hoc tests indicated that CS- startle was significantly higher for the maternal Lower Trauma class than the High Sexual Abuse class, Mdiff = 7.2 μV, 95 % CI [1.3, 13.1]. The maternal High Trauma and PTSD Symptoms class also had higher startle to CS- than either the Moderate Trauma class, Mdiff = 12.2 μV, 95 % CI [3.3, 22.8], or the High Sexual Abuse class, Mdiff = 10.7 μV, 95 % CI [2.2, 20.8]. See Fig. 4. As this analysis included age and violence exposure as covariates, the results indicate that the class differences in children’s startle to CS- was not driven by differences in age or violence exposure.
4. Discussion
This study of African American mothers and children with generally high levels of trauma and violence exposure examined whether there were distinct classes of mothers based on their trauma history and PTSD symptoms, and if there were distinct profiles of physiological response and fear learning in the children as a function of the mothers’ class membership. The LCA results indicated that within this sample, which has, on average, high levels of trauma exposure [61], there were four distinct classes of maternal trauma history and PTSD symptoms: Lower Trauma, Moderate Trauma, High Sexual Abuse, and High Trauma and PTSD Symptoms. Importantly, although the Lower Trauma class had the least childhood maltreatment and adult trauma exposure, their exposure was still high compared to less vulnerable populations: they reported some exposure childhood maltreatment and averaged more than three different types of adult trauma exposures. The classes differed in total exposure, but an additional factor that distinguished between classes was exposure to childhood sexual abuse. The Lower Trauma and High Sexual Abuse classes had similar levels of total childhood maltreatment, adult trauma, and PTSD symptoms; they differed only in the types of childhood maltreatment, and most clearly in their exposure to sexual abuse. The identification of these four classes within our sample of mothers provides a unique opportunity to examine how different patterns of maternal trauma exposure impact children.
The children completed a differential fear conditioning task while eye-blink startle responses were recorded; they acquired a conditioned fear response to the CS+, as well as discrimination between CS + and CS- over the course of the acquisition task, and the magnitude of fear responses to the CS- differed as a function of maternal class membership. There were significant class differences in startle to the safety signal (CS-), such that children of mothers in the Lower Trauma and High Trauma and PTSD Symptoms classes had larger fear responses than the children of mothers in the Moderate Trauma and High Sexual Abuse class. Children of the mothers in the High Sexual Abuse class had lower FPS to CS- than the Lower Trauma class. These results indicate while children acquired similar fear responses to the threat cue regardless of their mother’s trauma history, there were differences in safety signal learning based on maternal trauma history. Importantly, these findings were not explained by class differences in the children’s own developmental stage or violence exposure.
The FPS to the CS- during late acquisition provides an index of fear in the presence of a safety signal. The inability to appropriately inhibit fear responses to the CS- in the context of a fear conditioning task has been repeatedly linked to mental health outcomes [37,54,55,77–80]. Overall, inhibition of startle to the CS- along with amplification of startle to the CS + in the late phase of acquisition constitutes adaptive fear learning [37,53,79]. Our results indicate that children of mothers in the High Sexual Abuse class have blunted FPS to both CS types. In contrast, children of the Moderate Trauma mothers had the largest difference in their startle response to CS + versus CS-, and children of the Lower Trauma and High Trauma and PTSD Symptoms mothers had higher startle responses to CS- than the children of the High Sexual Abuse class mothers.
It is striking that children’s conditioned safety responses differed as a function of their mother’s trauma history and PTSD symptom severity. One prior study of adults with PTSD found that there were non-linear associations between amount of trauma exposure and startle responsivity in the context of aversive stimuli, such that exposure to multiple traumas was associated with blunted FPS magnitude and sympathetic nervous system responsivity during trauma imagery; Participants with a single trauma had significantly higher responses [81]. The study did not address whether type of trauma exposure, or an interaction of type and total exposure, might also impact physiological response. With respect to timing of trauma exposure, a study of adolescents with childhood maltreatment also found blunted fear conditioned responses to CS + and less discrimination between CS + and CS- [48]. Future studies should examine these factors in order to more fully elucidate the relationship between trauma, maltreatment, and subsequent risk to the individual and their offspring. Our results provide preliminary evidence that there may be complex effects of maternal trauma exposure type, timing, and magnitude on children’s ability to adaptively acquire and regulate fear responses.
Although we found that including age and violence exposure as covariates did not account for class differences in startle to CS-, it is important to consider the children’s developmental context. First, average violence exposure was high for children from all four maternal classes, with the average number of unique types of exposures ranging from 8–10. The children ranged from middle childhood to early adolescence, which is a period when significant shifts in fear learning have been documented. Prior studies have reported that children’s discrimination between threat and safety cues improves around age 10 [55,82], and that before age 10 children’s discrimination is moderated by maternal availability, such that children whose mother was present during acquisition discriminate more effectively than those without mother present [83]. Adding to the complexity of this developmental window, pubertal development is associated with longitudinal increases in FPS [80]. It is particularly striking that we observed significant maternal class differences in startle to the CS- in spite of the tremendous changes that unfold during this period of development. A remaining question is whether maternal trauma history impacts longitudinal change in their children’s fear learning, as well as associations between that change and the emergence of psychopathology.
The finding that childhood sexual abuse has distinct long-term effects on multiple outcomes is well-supported in the literature [2,84–86]. Childhood sexual abuse has been linked to blunting of HPA axis responsivity, measured as cortisol levels in response to a stressor [87]. One study of mothers with a history of MDD and their infants found that maternal child abuse history was associated with lower cortisol levels for the infant, compared to infants of non-abused mothers, and that these effects were more robust in mothers exposed to sexual versus physical abuse [29]. There is also evidence that maternal sexual abuse history may impact parental warmth, such that mothers are less warm towards their daughters but not their sons [88]. Relative to children whose mothers had either less maltreatment, other kinds of maltreatment, or extensive exposure to all types of maltreatment, our results indicate that children of mothers with high childhood sexual abuse exposure have blunted physiological responses, particularly to safety signals, in the context of a fear conditioning task.
We were not able to examine specific biological pathways through which maternal trauma history may have impacted the children to drive the class differences in fear responsivity and learning, however, several possibilities have been identified, as reviewed by [89,90]. Maternal childhood maltreatment has been associated with decreased grey matter volume in their infant children [20] and with higher levels of placental corticotropin-releasing hormone [56]. Two studies have reported that maternal trauma exposure is associated with lower cortisol levels in their infants [21,29]. One found that infants of mothers with childhood abuse exposure had lower cortisol levels than infants of unexposed mothers [29] and another found that maternal trauma exposure during pregnancy was associated with lower post-natal infant cortisol [21]. These results provide converging evidence that maternal trauma history impacts offspring via modulation of HPA axis function and, potentially, brain development. A critical next step is to elucidate these effects are related to intermediate risk phenotypes, including fear learning and inhibition.
These biological effects on physiological and brain development presumably interact with behavioral and environmental exposures as children develop. There is robust evidence that caregiving plays a central role in the development and regulation of the HPA axis [91–94].
Importantly, many studies have documented associations between maternal psychopathology and child development [32,95–98], and many of the mothers in our sample had high levels of PTSD symptoms. Children of parents with PTSD are at elevated risk for developing depression, anxiety, behavioral problems, and PTSD [22,23,99,100]. The mechanisms through which parental PTSD is transduced into elevated risk for psychopathology in their children is an area of active investigation, and the extent to which the trauma which led to PTSD versus the PTSD itself impacts children remains unclear. There is an outstanding need for additional research that unpacks the effects of parent’s trauma and mental health status on their children’s development and risk for psychopathology.
These findings make a novel contribution to the literature on intergenerational transmission of trauma by focusing on an intermediate phenotype associated with risk for fear and anxiety disorders, however, there were several limitations that should be addressed in future studies. First, because the number of mothers and children in each class were not evenly distributed, the small sample size in the Moderate Trauma and High Trauma and PTSD Symptoms classes precluded examination of possible sex differences within each class. Some prior studies report sex differences in the impact of maternal stress or trauma on offspring and the current results do not address this possibility. Second, we were not able to evaluate whether the class differences in children’s startle response were associated with neuroendocrine function, particularly the HPA axis, which has previously been associated with both intergenerational effects of trauma and startle responsivity. Third, this is a cross-sectional study that cannot address causation, whether these class differences persist as the children develop, or whether the class differences in fear learning as associated with psychopathology later in adolescence or in adulthood.
Our results provide novel evidence for intergenerational effects of maternal trauma on children’s startle responsivity in general, and particularly on their ability to inhibit fear in the presence of a safety signal. Although there were several limitations related to our study design and sample size, this finding is one of the first to examine how intergenerational transmission of trauma may impact an intermediate phenotype that has been repeatedly linked to risk for psychopathology. These results suggest that clinical assessments of children’s risk should consider maternal trauma history. There is a clear need for further study of how maternal trauma exposure confers risk via biological pathways, as well as how this impacts their children’s physiology, brain development, and cognitive processing in ways that may mediate their risk for psychopathology.
Acknowledgements
The authors thank Rebecca Hinrichs, Alisha Bhatia Compton, Sean Minton, Nadia Thompson, Aimee Clifford, and Charis Wiltshire for their contributions to data collection and preparation.
Funding
This work was supported by funding from the NIH (MH100122; MH111682) and Brain and Behavior Research Foundation.
Footnotes
CRediT authorship contribution statement
Anaïs F. Stenson: Conceptualization, Data curation, Formal analysis, Writing - original draft, Visualization. Sanne J.H. van Rooij: Data curation, Writing - review & editing. Sierra E. Carter: Data curation, Investigation, Writing - review & editing. Abigail Powers: Data curation, Investigation, Writing - review & editing. Tanja Jovanovic: Conceptualization, Funding acquisition, Writing - review & editing, Visualization.
References
- [1].Kessler RC, et al. , Trauma and PTSD in the WHO world mental health surveys, Eur. J. Psychotraumatol 8 (sup5) (2017), p. 1353383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Teicher MH, Samson JA, Anderson CM, Ohashi K, The effects of childhood maltreatment on brain structure, function and connectivity, Nat. Rev. Neurosci 17 (10) (2016) 652–666. [DOI] [PubMed] [Google Scholar]
- [3].Herringa RJ, et al. , Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence, Proc. Natl. Acad. Sci 110 (47) (2013) 19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brewin CR, The nature and significance of memory disturbance in posttraumatic stress disorder, Annu. Rev. Clin. Psychol 7 (1) (2011) 203–227. [DOI] [PubMed] [Google Scholar]
- [5].Stevens JS, et al. , Episodic memory after trauma exposure: medial temporal lobe function is positively related to re-experiencing and inversely related to negative affect symptoms, Neuroimage Clin. 17 (August) (2018) 650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Daskalakis NP, Yehuda R, Site-specific methylation changes in the glucocorticoid receptor exon 1F promoter in relation to life adversity: systematic review of contributing factors, Front. Neurosci 8 (October) (2014) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bustamante AC, et al. , Glucocorticoid receptor DNA methylation, childhood maltreatment and major depression, J. Affect. Disord 206 (2016) 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Norrholm SD, et al. , Fear load: the psychophysiological over-expression of fear as an intermediate phenotype associated with trauma reactions, Int. J. Psychophysiol 98 (2) (2015) 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wolf EJ, et al. , Traumatic stress and accelerated DNA methylation age: a meta-analysis, Psychoneuroendocrinology 92 (August) (2018) 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes katherine M., Friedman MJ , Prevalence using DSM-IV and DSM-5 criteria, J. Trauma. Stress 26 (5) (2013) 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Merrick MT, et al. , Vital signs: estimated proportion of adult health problems attributable to adverse childhood experiences and implications for prevention −25 states, 2015–2017, MMWR Morb. Mortal. Wkly. Rep 68 (44) (2019) 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shirtcliff EA, Coe CL, Pollak SD, Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1, Proc. Natl. Acad. Sci. U. S. A 106 (8) (2009) 2963–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V, Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α, Mol. Psychiatry 21 (5) (2016) 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Danese A, Tan M, Childhood maltreatment and obesity: systematic review and meta-analysis, Mol. Psychiatry 19 (5) (2014) 544–554. [DOI] [PubMed] [Google Scholar]
- [15].Mersky JP, Topitzes J, Reynolds AJ, Impacts of adverse childhood experiences on health, mental health, and substance use in early adulthood: a cohort study of an urban, minority sample in the U.S, Child Abus. Negl 37 (11) (2013) 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Heim C, et al. , Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood, JAMA 284 (5) (2000), p. 592. [DOI] [PubMed] [Google Scholar]
- [17].Jovanovic T, et al. , Exposure to violence accelerates epigenetic aging in children, Sci. Rep 7 (1) (2017), p. 8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weiss EL, Longhurst JG, Mazure CM, Childhood sexual abuse as a risk factor for depression in women: psychosocial and neurobiological correlates, Am. J. Psychiatry 156 (6) (1999) 816–828. [DOI] [PubMed] [Google Scholar]
- [19].Powers A, Fani N, Cross D, Ressler KJ, Bradley B, Childhood trauma, PTSD, and psychosis: findings from a highly traumatized, minority sample, Child Abus. Negl 58 (2016) 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moog NK, et al. , Intergenerational effect of maternal exposure to childhood maltreatment on newborn brain anatomy, Biol. Psychiatry 83 (2) (2018) 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, Berkowitz GS, Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy, J. Clin. Endocrinol. Metab 90 (7) (2005) 4115–4118. [DOI] [PubMed] [Google Scholar]
- [22].Yehuda R, Halligan SL, Grossman R, Childhood trauma and risk for PTSD: relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion, Dev. Psychopathol 13 (3) (2001) 733–753. [DOI] [PubMed] [Google Scholar]
- [23].van Ee E, Kleber RJ, Jongmans MJ, Relational patterns between caregivers with PTSD and their nonexposed children: a review, Trauma, Violence, Abus. 17 (2) (2015) 186–203. [DOI] [PubMed] [Google Scholar]
- [24].Dubowitz H, et al. , Type and timing of mothers’ victimization: effects on mothers and children, Pediatrics 107 (4) (2001) 728–735. [DOI] [PubMed] [Google Scholar]
- [25].Bosquet Enlow M, Devick KL, Brunst KJ, Lipton LR, Coull BA, Wright RJ, Maternal lifetime trauma exposure, prenatal cortisol, and infant negative affectivity, Infancy 22 (4) (2017) 492–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jovanovic T, et al. , Physiological markers of anxiety are increased in children of abused mothers, J. Child Psychol. Psychiatry 52 (8) (2011) 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yehuda R, Bell A, Bierer LM, Schmeidler J, Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors, J. Psychiatr. Res 42 (13) (2008) 1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yehuda R, et al. , Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring, Am. J. Psychiatry 171 (8) (2014) 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Brand SR, Brennan PA, Newport DJ, Smith AK, Weiss T, Stowe ZN, The impact of maternal childhood abuse on maternal and infant HPA axis function in the postpartum period, Psychoneuroendocrinology 35 (5) (2010) 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Danielson CK, Hankin BL, Badanes LS, Youth offspring of mothers with posttraumatic stress disorder have altered stress reactivity in response to a laboratory stressor, Psychoneuroendocrinology 53 (2015) 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bosquet Enlow M, Englund MM, Egeland B, Maternal childhood maltreatment history and child mental health: mechanisms in intergenerational effects, J. Clin. Child Adolesc. Psychol (2016) 1–16, vol. 00, no. 00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hartzell G, et al. , Intergenerational effects of maternal PTSD: Roles of parenting stress and child sex, Psychol. Trauma Theory, Res. Pract. Policy (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Coley EJL, et al. , Cross-generational transmission of early life stress effects on HPA regulators and bdnf are mediated by sex, lineage, and upbringing, Front. Behav. Neurosci 13 (May) (2019) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Costa DL, Yetter N, Desomer H, Intergenerational transmission of paternal trauma among US Civil War ex-POWs, PNAS 115 (44) (2018) 11215–11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chan JC, Nugent BM, Bale TL, Parental advisory: maternal and paternal stress can impact offspring neurodevelopment, Biol. Psychiatry 83 (10) (2018) 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cowan CSM, Callaghan BL, Kan JM, Richardson R, The lasting impact of early-life adversity on individuals and their descendants: potential mechanisms and hope for intervention, Genes Brain Behav. 15 (1) (2016) 155–168. [DOI] [PubMed] [Google Scholar]
- [37].Jovanovic T, Ressler KJ, How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD, Am. J. Psychiatry 167 (6) (2010) 648–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Franklin TB, et al. , Epigenetic transmission of the impact of early stress across generations, Biol. Psychiatry 68 (5) (2010) 408–415. [DOI] [PubMed] [Google Scholar]
- [39].Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL, Paternal stress exposure alters sperm MicroRNA content and reprograms offspring HPA stress axis regulation, J. Neurosci 33 (21) (2013) 9003–9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nephew BC, et al. , Intergenerational accumulation of impairments in maternal behavior following postnatal social stress, Psychoneuroendocrinology 82 (2017) 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dias BG, Ressler KJ, Parental olfactory experience influences behavior and neural structure in subsequent generations, Nat. Neurosci 17 (December) (2014) 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jovanovic T, et al. , Fear potentiation is associated with hypothalamic-pituitary-adrenal axis function in PTSD, Psychoneuroendocrinology 35 (6) (2010) 846–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Duits P, et al. , Updated meta-analysis of classical fear conditioning in the anxiety disorders, Depress. Anxiety 32 (4) (2015) 239–253. [DOI] [PubMed] [Google Scholar]
- [44].Yeomans JS, Li L, Scott BW, Frankland PW, Tactile, acoustic and vestibular systems sum to elicit the startle reflex, Neurosci. Biobehav. Rev 26 (1) (2002) 1–11. [DOI] [PubMed] [Google Scholar]
- [45].Davis M, Gendelman DS, Tischler MD, Gendelman PM, A primary acoustic startle circuit: lesion and stimulation studies, J. Neurosci 2 (6) (1982) 791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Walker DL, Davis M, The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction, Pharmacol. Biochem. Behav 71 (3) (2002) 379–392. [DOI] [PubMed] [Google Scholar]
- [47].Jovanovic T, Blanding NQ, Norrholm SD, Duncan E, Bradley B, Ressler KJ, Childhood abuse is associated with increased startle reactivity in adulthood, Depress. Anxiety 26 (11) (2009) 1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].McLaughlin KA, et al. , Maltreatment exposure, brain structure, and fear conditioning in children and adolescents, Neuropsychopharmacology 41 (8) (2016) 1956–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Grillon C, et al. , Families at high and low risk for depression: a three-generation startle study, Biol. Psychiatry 57 (9) (2005) 953–960. [DOI] [PubMed] [Google Scholar]
- [50].Waters AM, Peters RM, Forrest KE, Zimmer-Gembeck M, Fear acquisition and extinction in offspring of mothers with anxiety and depressive disorders, Dev. Cogn. Neurosci 7 (2014) 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pittig A, Treanor M, LeBeau RT, Craske MG, The role of associative fear and avoidance learning in anxiety disorders: gaps and directions for future research, Neurosci. Biobehav. Rev 88 (October) (2018) 117–140. [DOI] [PubMed] [Google Scholar]
- [52].Grillon C, Morgan CA, Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder, J. Abnorm. Psychol 108 (1) (1999) 134–142. [DOI] [PubMed] [Google Scholar]
- [53].Jovanovic T, et al. , Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity, Psychiatry Res. 167 (1–2) (2009) 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Waters A, Theresiana C, Neumann D, Craske M, Developmental differences in aversive conditioning, extinction, and reinstatement: a study with children, adolescents, and adults, J. Exp. Child Psychol 159 (2017) 263–278. [DOI] [PubMed] [Google Scholar]
- [55].Jovanovic T, et al. , Development of fear acquisition and extinction in children: Effects of age and anxiety, Neurobiol. Learn. Mem 113 (2014) 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Moog NK, et al. , Maternal exposure to childhood trauma is associated during pregnancy with placental-fetal stress physiology, Biol. Psychiatry 79 (10) (2016) 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Samuelson KW, et al. , Longitudinal effects of PTSD on memory functioning, J. Int. Neuropsychol. Soc 15 (6) (2009) 853–861. [DOI] [PubMed] [Google Scholar]
- [58].Jovanovic T, et al. , Cortisol suppression by dexamethasone reduces exaggerated fear responses in posttraumatic stress disorder, Psychoneuroendocrinology 36 (10) (2011) 1540–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Grillon C, Pine DS, Baas JMP, Lawley M, Ellis V, Charney DS, Cortisol and DHEA-S are associated with startle potentiation during aversive conditioning in humans, Psychopharmacology (Berl.) 186 (3) (2006) 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yehuda R, et al. , Holocaust exposure induced intergenerational effects on FKBP5 methylation, Biol. Psychiatry 80 (5) (2016) 372–380. [DOI] [PubMed] [Google Scholar]
- [61].Gillespie CF, et al. , Trauma exposure and stress-related disorders in inner city primary care patients, Gen. Hosp. Psychiatry 31 (6) (2009) 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kamkwalala A, et al. , Dark-enhanced startle responses and heart rate variability in a traumatized civilian sample: putative sex-specific correlates of posttraumatic stress disorder, Psychosom. Med 74 (2) (2012) 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bernstein DP, Fink L, Childhood Trauma Questionnaire Manual, San Antoinio, TX, 1998. [Google Scholar]
- [64].Bernstein DP, et al. , Development and validation of a brief screening version of the Childhood Trauma Questionnaire, Child Abuse Negl. 27 (2) (2003) 169–190. [DOI] [PubMed] [Google Scholar]
- [65].Schwartz AC, Bradley RL, Sexton M, Sherry A, Ressler KJ, Posttraumatic stress disorder among African Americans in an inner city mental health clinic, Psychiatr. Serv 56 (2) (2005) 212–215. [DOI] [PubMed] [Google Scholar]
- [66].Foa EB, Tolin DF, Comparison of the PTSD symptom scale-interview version and the clinician-administered PTSD scale, J. Trauma. Stress 13 (2) (2000) 181–191. [DOI] [PubMed] [Google Scholar]
- [67].Norrholm SD, et al. , Versatility of fear-potentiated startle paradigms for assessing human conditioned fear extinction and return of fear, Front. Behav. Neurosci 5 (November) (2011) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nylund KL, Asparouhov T, Muthén BO, Deciding on the number of classes in latent class analysis and growth mixture modeling: a monte carlo simulation study, Struct. Equ. Model. A Multidiscip. J 14 (4) (2007) 535–569. [Google Scholar]
- [69].Ayer L, Danielson CK, Amstadter AB, Ruggiero K, Saunders B, Kilpatrick D, Latent classes of adolescent posttraumatic stress disorder predict functioning and disorder after 1 year, J. Am. Acad. Child Adolesc. Psychiatry 50 (4) (2011) 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Stenson AF, et al. , Puberty drives fear learning during adolescence, Dev. Sci (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Norrholm SD, et al. , Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity, Biol. Psychiatry 69 (6) (2011) 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jovanovic T, et al. , Impact of ADCYAP1R1 genotype on longitudinal fear conditioning in children: interaction with trauma and sex, Neuropsychopharmacology (June) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Efron B, Tibshirani RJ, An Introduction to the Bootstrap, Chapman and Hall, New York, 1993. [Google Scholar]
- [74].Stine R, An introduction to bootstrap methods: examples and ideas, in: Fox J, Long JS (Eds.), Modern Methods of Data Analysis, Sage, Newbury Park, CA, 1990, pp. 325–373. [Google Scholar]
- [75].Wasserman S, Bockenholt U, Bootstrapping: applications to psychophysiology, Psychophysiology 26 (2) (1989) 208–221. [DOI] [PubMed] [Google Scholar]
- [76].Asparouhov T, Muthén B, Auxiliary variables in mixture modeling: three-step approaches using mplus, Struct. Equ. Model 21 (3) (2014) 329–341. [Google Scholar]
- [77].Pittig A, Treanor M, LeBeau RT, Craske MG, The role of associative fear and avoidance learning in anxiety disorders: gaps and directions for future research, Neurosci. Biobehav. Rev 88 (October) (2018) 117–140. [DOI] [PubMed] [Google Scholar]
- [78].Craske MG, et al. , Is aversive learning a marker of risk for anxiety disorders in children? Behav. Res. Ther 46 (8) (2008) 954–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jovanovic T, Norrholm SD, Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder, Front. Behav. Neurosci 5 (July) (2011) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Stenson AF, et al. , Puberty drives fear learning during adolescence, Dev. Sci (June) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Shumen JR, Bradley MM, Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity, Biol. Psychiatry 67 (4) (2010) 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Glenn CR, Klein DN, Lissek S, Britton JC, Pine DS, Hajcak G, The development of fear learning and generalization in 8–13 year-olds, Dev. Psychobiol 54 (7) (2012) 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].van Rooij SJH, et al. , Maternal buffering of fear-potentiated startle in children and adolescents with trauma exposure, Soc. Neurosci 12 (1) (2017) 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Negriff S, Blankson AN, Trickett PK, Pubertal timing and tempo: associations with childhood maltreatment, J. Res. Adolesc 25 (2) (2015) 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Cicchetti D, Rogosch FA, Gunnar MR, Toth SL, The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children, Child Dev. 81 (1) (2010) 252–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lewis T, McElroy E, Harlaar N, Runyan D, Does the impact of child sexual abuse differ from maltreated but non-sexually abused children? A prospective examination of the impact of child sexual abuse on internalizing and externalizing behavior problems, Child Abuse Negl. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Shenk CE, Noll JG, Putnam FW, Trickett PK, A prospective examination of the role of childhood sexual abuse and physiological asymmetry in the development of psychopathology, Child Abus. Negl 34 (10) (2010) 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Cross D, Kim YJ, Vance LA, Robinson G, Jovanovic T, Bradley B, Maternal child sexual abuse is associated with lower maternal warmth toward daughters but not sons, J. Child Sex. Abus 25 (8) (2016) 813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Rodgers AB, Bale TL, Germ cell origins of posttraumatic stress disorder risk: the transgenerational impact of parental stress experience, Biol. Psychiatry 78 (5) (2015) 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Chan JC, Nugent BM, Bale TL, Parental advisory: maternal and paternal stress can impact offspring neurodevelopment, Biol. Psychiatry 83 (10) (2018) 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, Nelson CA, Causal effects of the early caregiving environment on development of stress response systems in children, Proc. Natl. Acad. Sci. U. S. A 112 (18) (2015) 5637–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ, Moderate versus severe early life stress: associations with stress reactivity and regulation in 10–12-year-old children, Psychoneuroendocrinology 34 (1) (2009) 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Gunnar MR, DePasquale CE, Reid BM, Donzella B, Pubertal stress recalibration reverses the effects of early life stress in postinstitutionalized children, Proc. Natl. Acad. Sci. U. S. A 116 (48) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hostinar CE, Sullivan RM, Gunnar MR, Psychobiological mechanisms underlying the social buffering of the hypothalamic–pituitary–adrenocortical axis: A review of animal models and human studies across development, Psychol. Bull 140 (1) (2014) 256–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Bosquet Enlow M, Kitts RL, Blood E, Bizarro A, Hofmeister M, Wright RJ, Maternal posttraumatic stress symptoms and infant emotional reactivity and emotion regulation, Infant Behav. Dev 34 (4) (2011) 487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D, Maternal depression and child psychopathology: a meta-analytic review, Clin. Child Fam. Psychol. Rev 14 (1) (2011) 1–27. [DOI] [PubMed] [Google Scholar]
- [97].Muzik M, Morelen D, Hruschak J, Rosenblum KL, Bocknek E, Beeghly M, Psychopathology and parenting: an examination of perceived and observed parenting in mothers with depression and PTSD, J. Affect. Disord 207 (2017) 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Leen-Feldner EW, Feldner MT, Bunaciu L, Blumenthal H, Associations between parental posttraumatic stress disorder and both offspring internalizing problems and parental aggression within the National Comorbidity Survey-Replication, J. Anxiety Disord 25 (2) (2011) 169–175. [DOI] [PubMed] [Google Scholar]
- [99].Lambert JE, Holzer J, Hasbun A, Association between parents’ PTSD severity and children’s psychological distress: a meta-analysis, J. Trauma. Stress 27 (2014) 9–17. [DOI] [PubMed] [Google Scholar]
- [100].Chemtob CM, Nomura Y, Rajendran K, Yehuda R, Schwartz D, Abramovitz R, Impact of maternal posttraumatic stress disorder and depression following exposure to the september 11 attacks on preschool children’s behavior, Child Dev. 81 (4) (2010) 1129–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]


