Fig. 1. Schematic showing the mechanism of action of autophagy after cell injury.
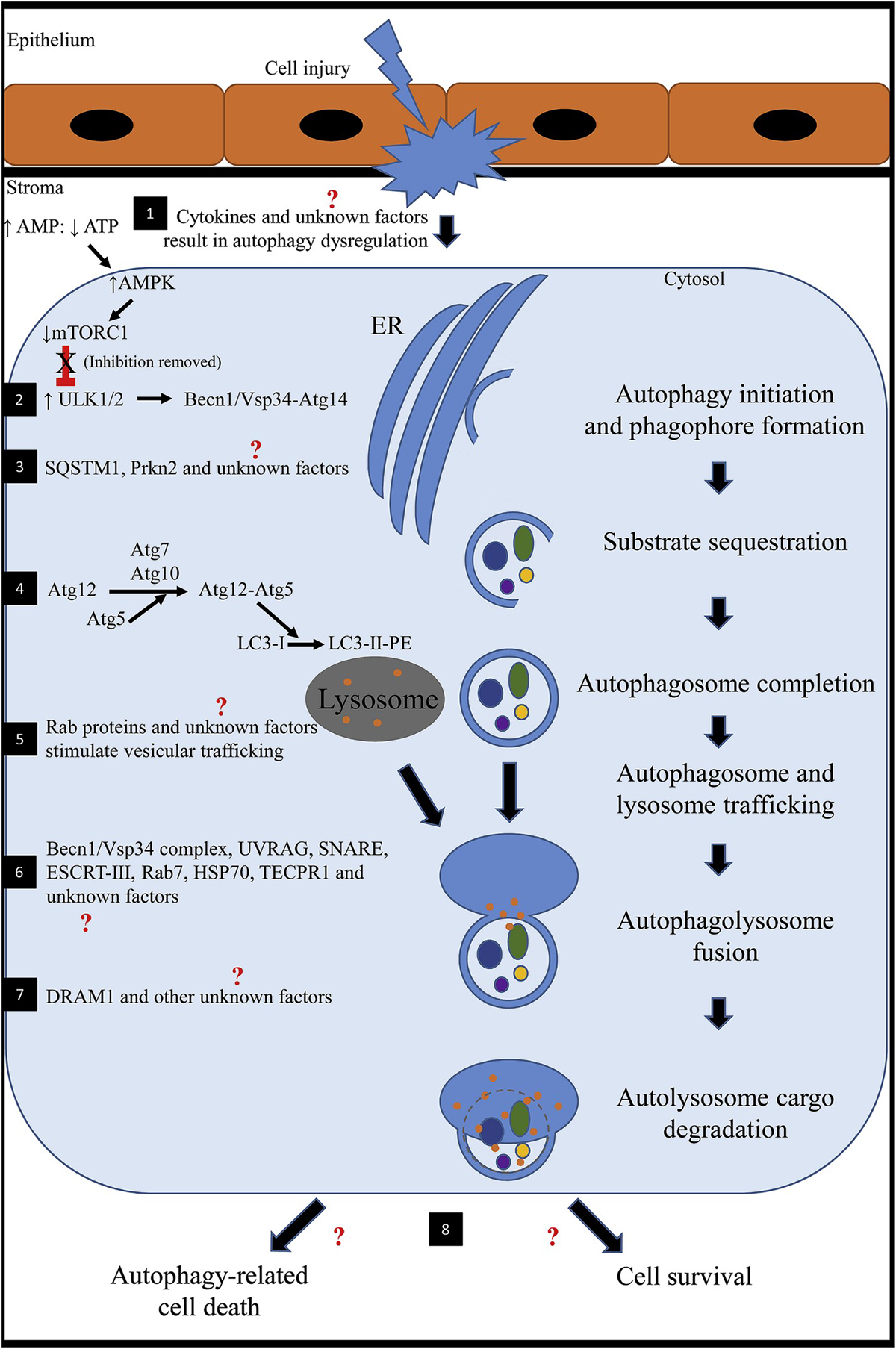
1) Following stress (injury to the corneal epithelium and underlying stroma), nutrient deprivation or hypoxia, cytokines (such as TNFα, IL-1, TGFβ) are released and initiate a cascade of signaling pathways. It is unknown how these factors specifically affect autophagy in the cornea. An altered ratio of AMP to ATP results in upregulation of AMPK. Increased AMPK inhibits mTOR1. In health, mTOR1 represses autophagy because there are sufficient nutrients for the cell to survive. AMPK-inhibition of mTOR1 activates ULK1 (mammalian homologue of Atg1). 2) ULK1 stimulates BECN1 to complex with Vsp34-Atg14 to regulate and initiate formation of the nascent autophagosome (phagophore) at the ER in response to stress signaling pathways. 3) SQSTM1, PRKN2 and other unknown factors selectively target obsolete organelles, misfolded proteins and fragmented/damaged organelles for degradation. 4) Atg7 and Atg10 facilitates Atg5 to conjugate with Atg12. The Atg12-Atg5 complex stimulates LC3-I (cytosolic form) conjugation to PE to form an LC3-II-PE complex that binds the surface of the expanding phagophore. Lipids are continually supplied until the autophagosomal membrane is a double-membraned enclosed structure. 5) The newly formed autophagosome and lysosome traffic toward one another for fusion. In general, Rab GTPases have been found to be involved with intracellular vesicular trafficking. Transport typically involves three steps including 1) budding of a vesicle from the donor membrane, 2) selective identification of the vesicle to the acceptor membrane and 3) docking and fusion with the selected membrane. Rab proteins have not been investigated concerning autophagy in the cornea. 6) A number of factors have been identified to be involved in autophagosome-lysosome fusion; however, many have not been identified in the cornea. Known factors include BECN1/Vsp34 complex, UVRAG, SNARE, ESCRT-III, Rab 7, HSP70 and TECPR1. 7) After fusion, the autolysosome undergoes a series of changes including acidification of the encircled environment and release of degradative enzymes. DRAM1 has been implicated and promotes the afore mentioned processes. 8) Ultimately, the degraded protein products are predominantly recycled into peptides and amino acids for use by the cell or the cell might undergo autophagy-related cell death. The latter is a somewhat similar process to apoptosis; however, autophagy-related cell death is not well understood and might be implicated in disease development.
(AMP: adenosine monophosphate; AMPK: 5′ AMP-activated protein kinase; Atg: autophagy-related protein; ATP: adenosine triphosphate; BECN1: Beclin 1; DRAM1: DNA damage regulated autophagy modulator 1; ER: endoplasmic reticulum; ESCRT: endosomal sorting complex required for transport; HSP70: heat shock protein 70; IL-1: interleukin 1; LC3: microtubule associated protein 1 light chain 3 alpha; mTORC1: mammalian target of rapamycin complex 1; PE: phosphatidylethanolamine; PRKN2: E3 ubiquitin-protein ligase parkin; Rab: ras-related GTP-binding protein; SNARE: soluble N-ethylmaleimide-sensitive fusion attachment protein receptors; SQSTM1: sequestosome 1; TECPR1: tectonin beta-propeller repeat containing 1; TGFβ: transforming growth factor beta; TNFα: tumor necrosis factor alpha; ULK1: unc-51-like kinase 1; UVRAG: ultraviolet irradiation resistance-associated gene; Vsp34: vesicular protein sorting 34).
