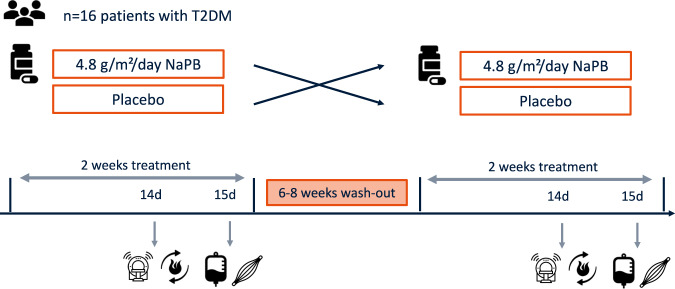
Fig. 1. Experimental design.
In this crossover study, participants were randomly assigned to start with 2-week NaPB supplementation or placebo treatment. After a washout period of 6–8 weeks, participants switched from intervention arm such that all participants served as their own control. In each treatment arm, measurements were performed after 2 weeks treatment, including magnetic resonance spectroscopy (day 14), whole-body 24 h energy metabolism and substrate oxidation (day 14), 2-step euglycemic hyperinsulinemic clamp (day 15) and muscle biopsies (day 15) were taken. T2DM patients with type 2 diabetes mellitus, NaPB sodium phenylbutyrate.