Figure 2.
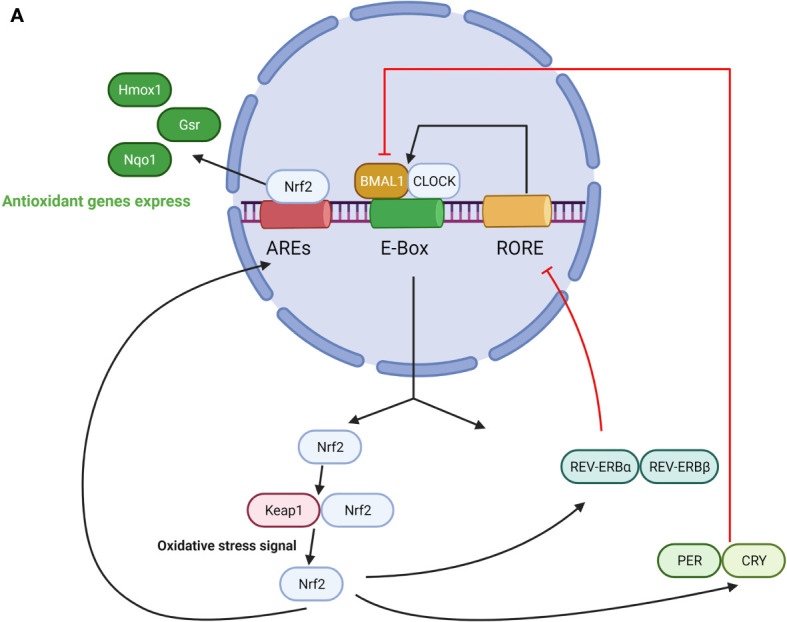
(A) The role of BMAL1 in the Nrf2 pathway. CLOCK/BMAL1 complexes exert temporal control on the E-box element in the Nrf2 gene promoter and positively regulate Nrf2 transcription. Nrf2 protein accumulates in a circadian manner and drives oscillations of ARE-regulated antioxidant genes. On the other hand, Nrf2 regulates the expression of PER, CRY, REV-ERBα/β, which represses CLOCK/BMAL1-regulated E-box transcription and switches off their production. (B) The role of BMAL1 in the NFκB pathway. On one hand, NFκB activation is under the regulation of a loop represented by the clock genes BMAL1/CLOCK, and their transcriptional positive and negative regulators REV-ERBα and RORα. Activation of this pathway leads to enhanced NAD+ levels and SIRT1 deacetylase activity, which in turn inactivates NFκB and reduces the binding ability to DNA. CLOCK upregulates NFκB–mediated transcription by increasing p65 phosphorylation and acetylation in the absence of BMAL1, and BMAL1 downregulates the CLOCK-dependent modulation of NFκB activation. On the other hand, NFκB antagonizes transcription of the CLOCK/BMAL1 target genes by directly binding to the promoters of the core clock repressors PER and CRY, which leads to highly specific transcriptional repression of CLOCK/BMAL1.
