Figure 4.
Exosomal non-coding RNAs and the immune tumor microenvironment
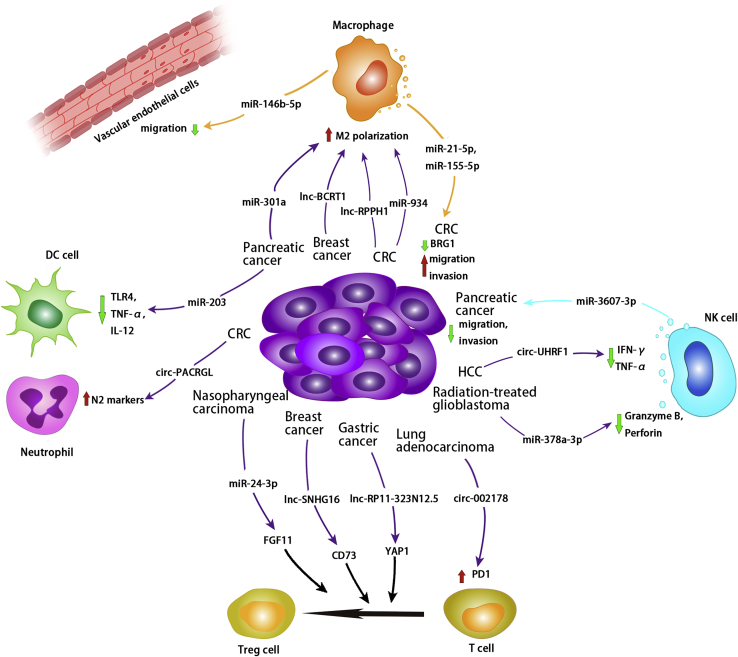
Exosomal non-coding RNAs from tumor cells can be internalized by macrophages and regulate their targets in macrophages, promoting M2 polarization in CRC, breast cancer, and pancreatic cancer cells. TAM-derived exosomal miR-21-5p and miR-155-5p downregulate BRG1, thereby promoting tumor migration and invasion in CRC. Macrophage-derived exosomal miR-146b-5p can inhibit vascular endothelial cell migration. Tumor-cell-derived exosomal non-coding RNAs regulate T cell differentiation into Treg cells in nasopharyngeal carcinoma, breast cancer, and gastric cancer. In addition, exosomal circ-002178 derived from lung adenocarcinoma has been shown to increase PD1 expression on T cells. Radiation-treated-glioblastoma-derived exosomal miR-378a-3p suppresses granzyme B and perforin expression in NK cells, whereas HCC-derived exosomal circ-UHRF1 suppresses IFN-γ and TNF-α expression in NK cells. However, exosomal miR-3607-3p derived from NK cells inhibits pancreatic tumor growth. Exosomal miR-203 derived from pancreatic cancer cells inhibits DC cell function by suppressing TLR4, TNF-α, and IL-12 expression. Exosomal circ-PACRGL derived from CRC cells increases N2 markers in neutrophils.