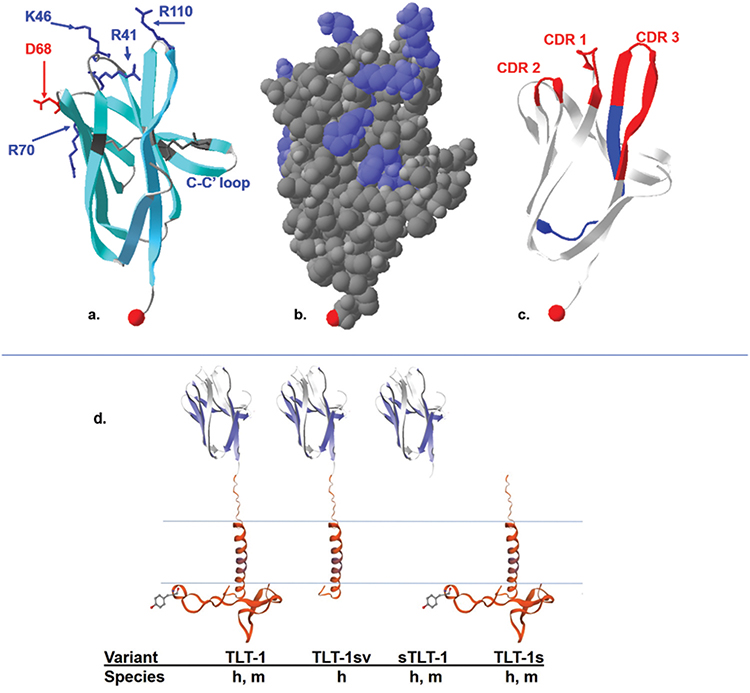
Figure 1.
The crystal structure of the TLT-1 extracellular domain (aa 20–125). a: ribbon model highlighting the negative residues in red, positive residues in blue, the two cysteine bonds characteristic of an Ig fold in dark gray. One of the disulfide bonds is from C38–C104, the second disulfide bond, connects C52 located in strand βC to C59 in strand βC’ (C-C’ loop). The C terminal region is represented by the red space filled proline. b: Space filled model showing the positively charged surface molecules (blue) over a relatively electrostatically neutral surface gray, the C-terminal region is represented by the light red space filed proline. c: The ribbon structure showing the CDR loops 1, 2, and 3 in red and the regions representing the LR12 peptide in blue. The C terminal region is represented by the red space filled proline. d: A schematic representation of the published TLT-1 isoforms with names listed below and designating that the TLT-1-sv is only found in humans (h); compared to the other forms that have been identified in mice(m) and humans. Figure was generated with the Swiss-PdbViewer 4.1.0, using data generated in DOI: 10.2210/pdb2FRG/pdb.