Figure 2.
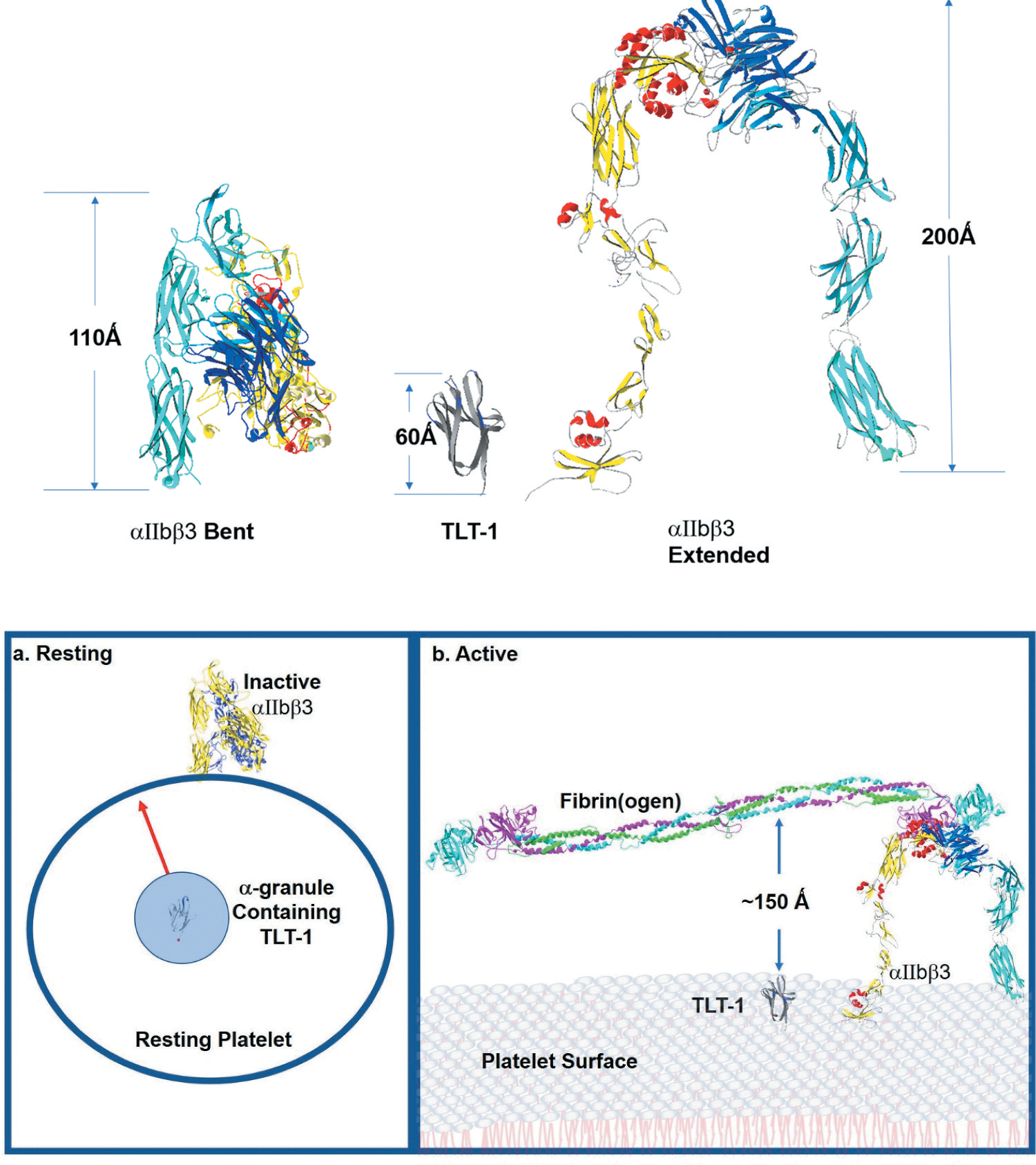
The platelet membrane landscape changes after platelet activation. Top - αIIbβ3 is the major platelet receptor. αIIbβ3 is a heterodimeric integrin receptor that binds fibrinogen. The α-subunit has 1039 amino acids and the β-subunit has 788 amino acids. On the resting platelets current models suggest that αIIbβ3 maintains an inactive bent (left) confirmation and upon platelet activation it changes from the bent form reaching to 110 Ǻ above the platelet surface to the extended form which towers to 200 Ǻ above the platelet surface (right). TLT-1 (middle) is estimated to reach between 50 and 60 Ǻ over the platelet surface. Bottom -. a: resting shows the relationship of TLT-1 to αIIbβ3 in resting platelets. The greater part of αIIbβ3 is found in the bent formation on the surface of resting platelets. There is some αIIbβ3 stored in the granules (not shown for clarity). TLT-1 on the other hand is sequestered in the α-granules and brought to the surface upon activation. On the resting platelet, neither receptor normally interacts with fibrinogen. Active(b) shows the surface of activated platelets. αIIbβ3 interacting with fibrin on the surface of a platelet showing TLT-1 for size comparison. Once on the platelet surface TLT-1 seems dwarfed in comparison to αIIbβ3. αIIbβ3 stands approximately 200 Ǻ over the platelet surface where TLT-1 is between 50–60 Ǻ including the stalk (not shown). Figure was generated with the Swiss-PdbViewer 4.1.0, using data published in DOI: 10.2210/pdb2FRG/pdb, DOI: 10.2210/pdb3FCS/pdb, DOI: 10.2210/pdb3GHG/pdb, and DOI: 10.2210/pdb4G1M/pdb.
