FIGURE 2.
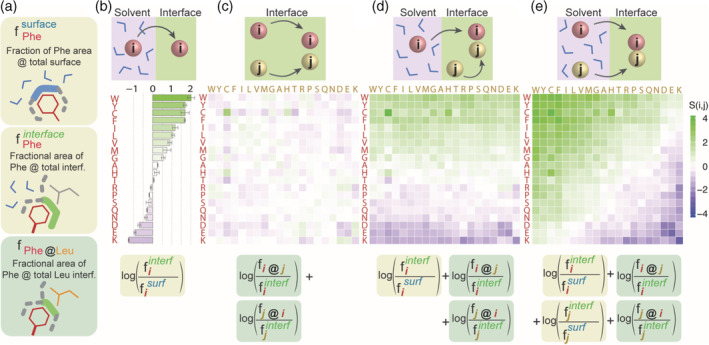
Defining amino acid interface propensities and interaction propensities based on surface areas. (a) We calculate three types of surface areas to derive interface propensities and pairwise residue contact propensities: (i) The area fraction an amino acid occupies at solvated surfaces. Phenylalanine, for example, makes up 1.33% of all protein surfaces in our dataset. (ii) The area fraction an amino acid occupies at interfaces. Phenylalanine, for example, makes up 7.19% of all protein interfaces in our dataset. (iii) The area fraction an amino acid makes up at a subinterface region defined by a particular amino acid. For example, phenylalanine makes up 8.17% of the total leucine interface area. (b) We estimate the free energy of transfer of amino acids from solvent to interface from the statistics of surface areas contributed to both regions. For example, the interface propensity of phenylalanine is log(0.0719/0.0133) = 1.69. (c) We estimate the interaction propensity of amino acids independently of their desolvation component. While the area fraction of phenylalanine at the total interface is 7.19%, it contacts 8.17% of leucine's interface area, highlighting a representation of this contact that is close to a random expectation: log(0.0817/0.0719) = 0.13. (d) We estimate the interaction propensity of amino acids with amino acid i being desolvated (red) and amino acid j (yellow) being already at the interface. (e) Interaction propensity that includes the desolvation component for both amino acids i and j
