Abstract
Introduction
Mid‐life dietary patterns are associated with Alzheimer's disease (AD) risk, although few controlled trials have been conducted.
Methods
Eighty‐seven participants (age range: 45 to 65) with normal cognition (NC, n = 56) or mild cognitive impairment (MCI, n = 31) received isocaloric diets high or low in saturated fat, glycemic index, and sodium (Western‐like/West‐diet vs. Mediterranean‐like/Med‐diet) for 4 weeks. Diet effects on cerebrospinal fluid (CSF) biomarkers, cognition, and cerebral perfusion were assessed to determine whether responses differed by cognitive status.
Results
CSF amyloid beta (Aβ)42/40 ratios increased following the Med‐diet, and decreased after West‐diet for NC adults, whereas the MCI group showed the reverse pattern. For the MCI group, the West‐diet reduced and the Med‐diet increased total tau (t‐tau), whereas CSF Aβ42/t‐tau ratios increased following the West‐diet and decreased following the Med‐diet. For NC participants, the Med‐diet increased and the West‐diet decreased cerebral perfusion.
Discussion
Diet response during middle age may highlight early pathophysiological processes that increase AD risk.
Keywords: Alzheimer's disease, cerebral perfusion, cerebrospinal fluid biomarkers, diet intervention
1. INTRODUCTION
Conditions associated with metabolic dysregulation, such as obesity, hypertension, type 2 diabetes, and hypercholesterolemia, have increased in prevalence in part due to rising consumption of saturated fats (SF), simple carbohydrates, and sodium, characteristic features of the “Western diet.” Such conditions associated with the Western dietary pattern may also promote pathological brain aging; for example, type 2 diabetes may decrease cerebral perfusion 1 and is associated with increased risk for Alzheimer's disease (AD) and other dementias. 2 Consumption of foods high in SF, glycemic index (GI), and sodium may induce hypercholesteremia and insulin resistance (IR), which disrupt vasoreactivity, hemodynamic function, and endothelial integrity, 3 thereby impeding cerebral perfusion. Vascular dysfunction has long been considered a contributing factor to dementia, including but not limited to dementia due to AD. 4 Human brain imaging studies support this notion, showing that poor metabolic and vascular health and lower cerebral perfusion often precede development of AD. 5
Epidemiological studies provide evidence for a relationship between dietary patterns and AD, showing that high intake of SF and simple carbohydrates is associated with increased risk of AD 6 whereas diets low in SF and simple carbohydrates are associated with lower AD risk. 7 , 8 Deleterious effects of SF and high GI foods on vascular health arise, at least in part, due to hyperglycemia, hyperinsulinemia, and dyslipidemia that provokes IR. Such effects may also result in hypertension, a clinical manifestation of IR‐induced endothelial dysfunction. 9 The common feature of IR among vascular risk factors suggests that the interface between IR and vascular function may be a critical mechanistic pathway, that when disrupted may increase risk for AD and vascular dementia (VaD). Rodent studies examining this pathway show that high‐SF and high‐sucrose diets impair brain insulin signaling, impede endothelium‐dependent vasodilation, reduce capillary recruitment and microvascular flow, and increase AD‐related brain pathology. 10 , 11 , 12 , 13 , 14 , 15
Most evidence examining relationships between dietary pattern and AD risk and pathology in humans has been obtained from epidemiologic studies. Randomized trials may complement findings from such studies and elucidate the effects of diet on metabolic health, the aging brain, and AD pathology. In previous studies, diets high in SF and simple carbohydrates affected cognition and levels of cerebrospinal fluid (CSF) biomarkers in normal older adults and adults with mild cognitive impairment (MCI), a presumed prodrome of AD. 16 , 17 , 18 Further investigation of these issues may be critical in middle‐aged individuals when brain health may be most amenable to lifestyle intervention. To our knowledge, no study to date has examined the impact of a Western‐like diet on AD pathology, vascular function, and cognition in middle‐aged adults using a randomized controlled diet intervention. Further, no study has examined whether the effects of a Western‐like diet for adults with MCI differ than those experienced by normal cognition (NC) adults.
To address this gap, the present study compared effects of two isocaloric diets, a Western‐like diet (West‐diet) and a Mediterranean‐like diet (Med‐diet) and determined whether effects differed for NC adults or adults with MCI between 45 and 65 years of age, an age range conventionally referred to as middle‐age or mid‐life. The West‐diet intervention conformed to the macronutrient pattern often described as a “Western diet” generally associated with obesity, dyslipidemia, IR, and type 2 diabetes. In contrast, the Med‐diet was based on a “Mediterranean diet” profile associated with reduced risk for metabolic and cardiovascular disease. In this exploratory study, we compared the effects of the diet intervention on metabolic parameters, CSF AD biomarkers, cerebral perfusion assessed with magnetic resonance imaging (MRI), and cognition. We observed striking differences between MCI and NC groups in response to diet that may highlight key early pathophysiological processes.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed relevant literature with PubMed using search terms related to diet effects on metabolism, cognition, and cerebral blood flow (CBF) or the relationship of these factors with Alzheimer's disease (AD).
Interpretation: Our findings suggest that controlled 4‐week Western‐like and Mediterranean‐like diet interventions differentially impact metabolic factors, CBF, memory, and cerebrospinal fluid biomarkers associated with AD, and that effects may be modulated by cognitive status.
Future directions: Our results provide a framework for longer, larger intervention studies investigating the relationship between diet composition and AD symptoms and pathology, the potential use of a Mediterranean‐like diet during mid‐life as a strategy to promote healthy brain aging, and the need to understand mechanisms underlying differential responses to diet intervention in adults with mild cognitive impairment (MCI).
HIGHLIGHT
Mediterranean‐like diets had beneficial effects on cerebrospinal fluid AD biomarkers, cerebral blood flow, and memory for cognitively normal adults, whereas Western‐like diets had negative effects
Adults with MCI showed a different pattern from cognitively normal adults, with the Western‐diet producing favorable effects on cerebrospinal fluid biomarkers
The four‐week diet interventions had powerful effects on AD pathology and cerebrovascular indices, supporting the need for longer studies to examine diet as a preventative or therapeutic tool
2. MATERIALS AND METHODS
2.1. Participants
Institutional review boards IRBs at Wake Forest School of Medicine and the VA Puget Sound Health Care System (VAPSHCS) approved the current study (NCT02463084). Eighty‐seven middle‐aged adults were randomized and tested in clinical research units at either the Wake Forest School of Medicine in Winston‐Salem, North Carolina, or the VAPSHCS in Seattle, Washington. Adults were between 45 and 65 years of age and were recruited from the surrounding communities. Participants received cognitive and clinical evaluation and NC adults (n = 56) or adults with MCI (n = 31) adjudicated by an expert panel of physicians and neuropsychologists using National Institute on Aging–Alzheimer's Association criteria were enrolled. 19 Exclusion criteria included hypertension (blood pressure > 140/90), hyperlipidemia with or without treatment, current or previous use of diabetes medications, or current use of antihypertensive medications or cholesterol‐lowering medications. Individuals were also excluded if they had clinically significant elevations in liver function tests or lipids; major digestive disorders; significant neurological disease that might affect cognition; significant medical illness or organ failure; current use of antipsychotic, antidepressant, anticonvulsant, anticoagulant, anxiolytic, or sedative medications; current use of cognition‐enhancing medications or glucocorticoids.
2.2. Design and procedure
2.2.1. Diet groups
Participants were screened with physical exam, venipuncture, and cognitive testing 2 weeks prior to starting experimental diets. Eligible participants were then randomly assigned to the Med‐diet or West‐diet group. Investigators, participants, and all study personnel responsible for data collection were blind to dietary condition. Caloric assignment within each dietary condition was determined on an individual basis using basal metabolic rate (BMR) calculated using the Mifflin St. Jeor equation. 20 To ensure that body weight remained stable in both dietary conditions during the 4‐week intervention participants were asked to complete a 7‐day physical activity log and a 3‐day food record (2 weekdays and 1 weekend day), which were used to adjust BMR.
Diets differed in the quantity of SF and salt, and type of carbohydrate as measured by GI, to yield a high‐SF, high‐GI, high‐salt (Na+) (West‐diet), and a low‐SF, low‐GI, low‐Na+ diet (Med‐diet). The Med‐diet was modeled on a Mediterranean diet in that prepared meals used protein sources low in saturated fats (fish, lean meats), and incorporated healthy fats, plentiful fruits and vegetables, and whole grains. Participants were also allowed to consume one glass of wine per day. The Med‐diet was comprised of ≈40% total fat (< 7% of total calories from saturated fat), 40% to 45% of total calories from carbohydrates, with a mean daily GI < 55, 15% to 20% of calories from protein, and ≈1300 mg/day Na+ per 2000 calories (adjusted for calorie intake at 0.65 mg Na+ per calorie). The West‐diet contained ≈40% to 45% total fat (25% saturated fat), 40% carbohydrates (GI > 70), and 15% to 20% protein, and average sodium intake of ≈3200 mg/day for 2000 calories (adjusted for calorie intake at 1.6 mg Na+ per calorie). Meals for both dietary conditions were planned by a registered dietitian and prepared by a metabolic kitchen. Menus were designed to mask diet conditions. During the 4‐week intervention participants received bi‐weekly supplies of frozen entrees and other food items. Compliance was assessed by daily food journals where participants recorded food intake including weight of pre‐ and post‐consumption of meals. Participants were also contacted once a week to confirm compliance. After the completion of week 4, participants returned to the clinic to repeat collection of all measures acquired prior to diet initiation.
2.2.2. Lumbar puncture
Participants underwent lumbar puncture (LP) for collection of CSF in the morning after a 12‐hour fast. LP was conducted in the lateral decubitus position using a 22‐ or 24‐gauge Sprotte atraumatic needle inserted between the L3‐4 or L4‐5 vertebrae. Up to 25 mL of CSF was extracted into sterile polypropylene tubes, aliquoted into 0.2 mL pre‐chilled polypropylene tubes, frozen immediately on dry ice, and stored at –70°C until assay. CSF was assayed for amyloid beta (Aβ)42, Total tau (t‐tau) and phosphorylated tau (p‐tau)181 using a Luminex based INNO‐BIA Alzbio3 assay and CSF Aβ40 was measured by standard enzyme‐linked immunosorbent assay (ELISA) according to manufacturer instructions, in each case using kits from Fujirebio (formerly Innogenetics).
2.2.3. Blood biomarkers
Blood lipids, insulin, glucose, and HbA1c were measured in the morning after a 12‐hour fast by Laboratory Corporation of America (LabCorp). Apolipoprotein E (APOE) genotyping was conducted by the Genetics Core of Wake Forest University School of Medicine.
2.2.4. Cognitive tests
The Modified Mini‐Mental State Examination (3MS) was administered at baseline prior to dietary intervention to provide a global estimate of cognitive function. 21 Cognitive testing was conducted pre‐ and post‐diet intervention and included tests of delayed episodic memory (story recall 22 and Buschke Selective Reminding Test 23 ), and the Dot Counting Test of executive function. 24 Alternate forms of memory measures were administered in randomized order. A cognitive composite score was constructed by averaging Z scores for delayed episodic memory tests, and then averaging this score with a Z score calculated total number of correct trials for the Dot Counting Test. The use of the cognitive composite was based on evidence that a composite that includes both executive and episodic memory components may provide more a sensitive measure of possible diet effects on AD pathology for adults with NC or MCI. 25
2.3. Magnetic resonance imaging
Participants underwent MRI with a 3T Siemens Skyra scanner and a 32 channel head coil (Siemens). MRI high‐resolution T1‐weighted structural images were obtained using an MP‐RAGE sequence: TR = 2300; TI = 900; TE = 2.95 msec; 1 mm isotropic resolution. Pseudo‐continuous arterial spin labeling MRI (pcASL MRI) was performed to assess cerebral blood flow (CBF) with a whole brain MP‐pcASL sequence 26 using the following parameters: tagging duration = 1.7 sec, TI = 3.2 sec, TR = 4 sec, TE = 12 ms, reps = 81, FOV = 22 × 22 cm, twenty‐four 5 mm axial slices with a single shot EPI acquisition, collecting eight cycles where each cycle consists of eight images acquired with unique phase offsets, GRAPPA factor of two, acquisition time = 5 mins and 32 sec. The ASL time series were motion‐corrected and motion‐corrupted images were filtered using an algorithm 27 before averaging. The ASL images were quantified using a kinetic model described in Buxton et al. 28 and the quantified CBF was converted to absolute units (mL/100 g/min) using the CSF image as a reference signal. 29 This results in a calibrated perfusion value for each voxel. The CBF maps were co‐registered with the T1‐weighted structural images, normalized into Montreal Neurological Institute space for group analysis. Scans were examined for quality indices including motion artifact and excessive transit time. Scans from 49 participants with complete data that passed quality control inspections were included in a whole brain voxel‐wise analysis.
2.4. Compliance
Compliance was assessed through daily food records reviewed by a registered dietitian. Average number of non‐compliant meals per week was calculated for each participant.
2.5. Statistical analyses
Analyses were conducted with SAS (version 9.4). Body weight, total cholesterol, low‐density lipoproteins (LDL), high‐density lipoproteins (HDL), fasting glucose, fasting insulin, and HbA1c were subjected to repeated measures analysis of covariance (RMANCOVAs) in SAS (version 9.4), with diet group (Med‐ and West‐diet) and diagnosis (NC and MCI) as independent factors; time (pre‐ and post‐diet) as a repeated factor; and age, sex, baseline body mass index, baseline 3MS scores, APOE ɛ4 status (no ɛ4 alleles/negative vs. one or two alleles/positive), and site as covariates. Non‐contributory covariates (P < .15) were eliminated from the model. Interactions among diet, diagnosis, and time were examined to test the hypothesis that the effects of diet would differ for the NC and MCI groups. Given the exploratory nature of the study, the direction of significant and trend‐level interactions was investigated in post‐hoc analyses without correcting for multiple comparisons. Only participants with complete baseline and post‐diet data were included in analyses. The same approach was used to examine diet effects on CSF AD biomarkers (Aβ42, Aβ40, t‐tau, p‐tau181, and the ratios Aβ42/40 and Aβ42/t‐tau), the cognitive composite Z score, and compliance estimates.
For pcASL MRI, voxel‐wise analyses were conducted in SPM12 to compare cerebral perfusion for the Med‐ and West‐ diets. An a priori anatomical mask was constructed using the WFU PickAtlas toolbox 30 and applied to all analyses. The mask was composed of 25 bilateral AAL regions (see Figure 4A; Table S1 in supporting information) shown to be negatively affected by AD, 31 including: bilateral superior, inferior, middle, and medial frontal cortices; superior, middle, and inferior temporal gyrus; posterior cingulate; precuneus; parahippocampal gyrus; amygdala; and hippocampus. Data were corrected for multiple comparisons with a voxel‐wise threshold level of P < .01 holding alpha at 0.01 for a minimum cluster size of 205 contiguous voxels. This a priori threshold was derived via Monte Carlo simulations (3dClustSim, AFNI, https://afni.nimh.nih.gov). To examine regions of significance observed in voxel‐wise analysis, contrast beta weights were extracted from significant clusters (average of all voxels from significant clusters) for both pre‐ and post‐diet pcASL MRI scans for both diet types using MarsBaR (version 0.44), yielding an index of mean cerebral perfusion across significant voxels. This mean cerebral perfusion index was subjected to RMANCOVA. Diet and diagnosis were the between‐subject factors; time (pre‐ and post‐diet) was the repeated factor; and age, sex, 3MS, APOE ɛ4 status, and test site were included as covariates.
FIGURE 4.
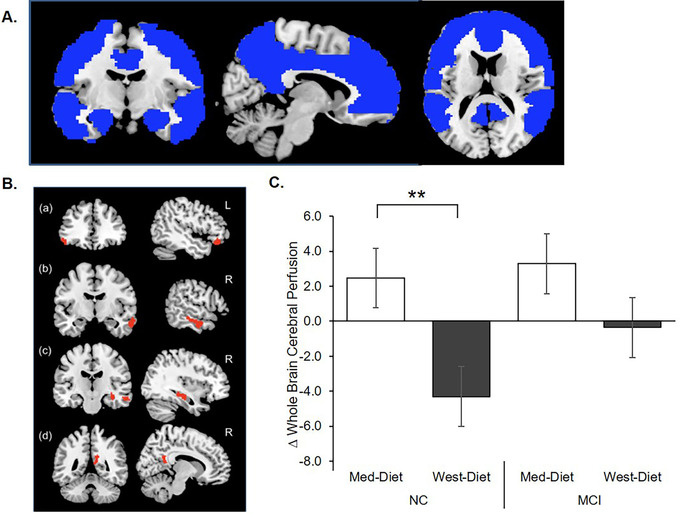
Diet effects on cerebral perfusion assessed with pseudo‐continuous arterial spin labeling (pcASL) magnetic resonance imaging (MRI). A, Rendering of the a priori anatomical mask used in the study constructed using WFU PickAtlas toolbox. 30 The mask included the bilateral superior, inferior, middle and medial frontal cortices; superior, middle, and inferior temporal gyrus; posterior cingulate, precuneus, parahippocampal gyrus; amygdala; and hippocampus. B, The Mediterranean diet (Med‐diet) was associated with significantly greater cerebral perfusion following diet intervention compared to the Western diet (West‐diet) group in the (a) left inferior frontal cortex and the right temporal lobe (b), hippocampus (c), and precuneus (d). C, Normal cognition (NC) participants showed increased mean cerebral perfusion after the Med‐diet, and decreased perfusion after the West‐diet (P = .003). Although the mild cognitive impairment (MCI) group perfusion values also increased following the Med‐diet, this effect was variable and did not approach significance (P = .499). Figures depict change scores for ease of interpretation, adjusted means from repeated measures analysis of covariance are included in Table S2. Error bars represent ± 1 standard error from the mean. Significance is set at + P < .10, *P < .05, ** P < .01, or *** P < .001
3. RESULTS
3.1. Participants
A total of 157 participants were screened for the study, 70 of whom did not meet eligibility requirements, resulting in 87 participants who were randomized on a 1:1 basis to the Med‐diet (n = 44) or West‐diet (n = 43) arm (Figure 1). The main reasons for screen failure included hypertension or other exclusionary medical conditions, or prospective participants changing their mind about participating after receiving detailed information about mandatory study procedures. Forty‐one participants completed the Med‐diet arm and 43 participants completed the West‐diet arm. There were no differences in demographic characteristics or in body mass index between assigned diet groups (Table 1). The MCI group (n = 31, mean age 56.2 ± 5.1 years) had lower 3MS scores than the NC group (n = 56, mean age 56.3 ± 5.1 years; P = .007), with no other significant demographic differences noted.
FIGURE 1.
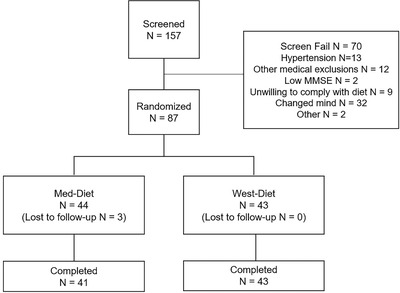
Consort diagram of study enrollment
TABLE 1.
Baseline descriptive characteristics
| NC | MCI | |||
|---|---|---|---|---|
| Med‐diet | West‐diet | Med‐diet | West‐diet | |
| N (female) | 31 (21) | 25 (19) | 13 (8) | 18 (10) |
| Age, years | 55.77 ± 5.6 | 57.0 ± 4.4 | 55.46 ± 5.3 | 56.72 ± 5.0 |
| Education, years | 15.55 ± 2.1 | 16.04 ± 1.7 | 14.46 ± 2.5 | 15.33 ± 2.8 |
| BMI (kg/m2) | 28.23 ± 5.2 | 27.63 ± 6.5 | 28.68 ± 4.4 | 27.37 ± 4.3 |
| 3MS scorea | 97.13 ± 2.6 | 97.62 ± 2.1 | 94.31 ± 5.9 | 96.33 ± 2.5 |
aMCI < NC, P = .007
Notes: Baseline descriptive characteristics for NC and MCI groups consuming the Med‐ and West‐diets. Data are reported as mean ± standard deviation.
Abbreviations: 3MS, Modified Mini‐Mental Status Examination; BMI, body mass index; MCI, mild cognitive impairment; Med‐diet, Mediterranean diet; NC, normal cognition; West‐diet, Western diet.
3.2. Effects of diet on lipid and metabolic markers
The West‐diet increased and the Med‐diet reduced total cholesterol levels for both NC and MCI groups similarly (diet x time F[1,69] = 21.89, P = .0001; Figure 2A). This pattern was also observed for LDL and HDL cholesterol (diet x time F[1,68] = 26.54, P = .0001 for LDL; diet x time F[1,72] = 13.07, P = .0006 for HDL; Figure 2B‐C).
FIGURE 2.
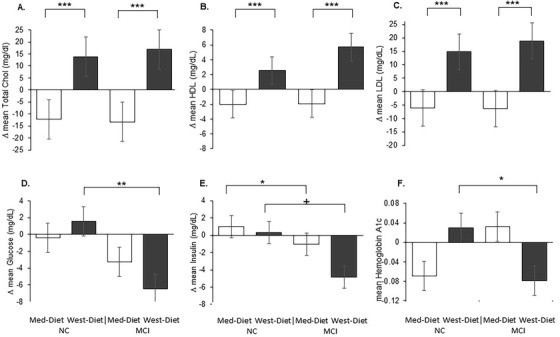
Diet effects on peripheral metabolism. A‐C) Total cholesterol, low‐density lipoprotein (LDL), and high‐density lipoprotein (HDL) levels were significantly reduced following the Mediterranean diet (Med‐diet) and increased following the Western diet (West‐diet) for both normal cognition (NC) and mild cognitive impairment (MCI) groups (all Ps < .001). D, Plasma glucose levels increased for the NC group and decreased for the MCI group following the West‐diet (P = .01), with no changes observed for groups following the Med‐diet. E Plasma insulin levels increased for the NC group and decreased for the MCI group following Med‐diet (P = .031). Insulin also tended to decrease for the MCI but not the NC group during the West‐diet (P = .051). F, The NC group showed increased HbA1c levels following the West‐diet, and the MCI group showed decreased levels (P = .049). Figures depict change scores for ease of interpretation, adjusted means from repeated measures analysis of covariance are included in Table S2. Error bars represent ± 1 standard error from the mean. Significance is set at + P < .10, *P < .05, ** P < .01, or *** P < .001
NC and MCI groups showed different responses to the diet for fasting plasma glucose levels (diagnosis x time F[1,71] = 7.82, P = .007). Both groups’ levels were unchanged following the Med‐diet, whereas following the West‐diet, the NC group's levels increased and the MCI group's levels decreased (diagnosis x time F[1,34] = 7.47, P = .010; Figure 2D). For fasting insulin levels, the NC group increased and the MCI group decreased following the Med‐diet; the MCI group insulin levels also decreased during the West‐diet, whereas the NC group showed little change (diagnosis x time F[1,72] = 6.72, P = .012; diagnosis x time for West‐diet F[1,35] = 4.07, P = .051; diagnosis x time for the Med‐diet F[1,37] = 5.01, P = .031; Figure 2E). A three‐way interaction of diet x cognitive group x time was observed for HbA1c (F[1,69] = 4.67, P = .034); the NC group showed increased HbA1c levels following the West‐diet, and the MCI group showed decreased levels (F[1,36] = 4.17, P = .049; Figure 2F), whereas the opposite, though non‐significant, pattern was observed for the Med‐diet.
3.3. Diet group and CSF AD biomarkers
Diet affected Aβ40 levels differently for NC and MCI groups (diagnosis x time F[1,59] = 4.25, P = .044; diet x time x cognitive group F[1,59] = 3.24, P = .077); the NC group showed decreased Aβ40 levels following the Med‐diet, and increased levels after the West‐diet (F[1,41] = 5.33, P = .026), whereas the MCI group levels were unchanged (P = .469; Figure 3A). No effects of diet were observed for CSF Aβ42 (mean pre‐ and post‐ Med‐diet levels for NC and MCI = 417.0 ± 40.5 and 399.8 ± 37.9 vs. 444.0 ± 53.6 and 395.7 ± 50.2; mean pre‐ and post‐West diet levels for NC and MCI = 498.8 ± 39.6 and 457.9 ± 37.0 vs. 397.1 ± 51.4 and 392.8 ± 48.2). However, a significant three‐way interaction of diet x cognitive group x time was observed for the CSF Aβ42/40 ratio (F[1,64] = 9.57, P = .003); the NC group showed increased ratios in following the Med‐diet, and reduced ratios after the West‐diet (diet group x time F[1,41] = 6.59, P = .014; Figure 3B), whereas the MCI group showed a trend for the reverse pattern (F[1,23] = 3.78, P = .064).
FIGURE 3.
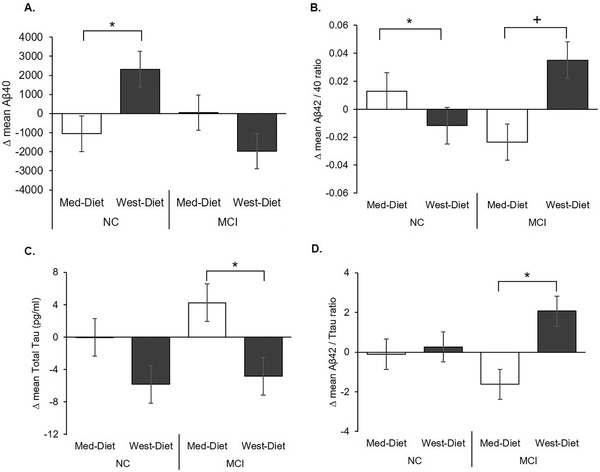
Diet effects on cerebrospinal fluid (CSF) Alzheimer's disease (AD) biomarkers. A, CSF amyloid beta (Aβ)40 decreased for normal cognition (NC) participants following the Mediterranean diet (Med‐diet) and increased following the Western diet (West‐diet; P = .026). B, The CSF Aβ42/40 ratio increased for NC participants following the Med‐diet and decreased after the West‐diet (P = .014), and the mild cognitive impairment (MCI) participants showed a trend for the reverse pattern (P = .064). C, Total tau (t‐tau) was increased by the Med‐diet and reduced by the West‐diet for the MCI group (P = .044). D, The Aβ42/t‐tau ratio was reduced following the Med‐diet for the MCI group and increased following the West‐diet (P = .036). Figures depict change scores for ease of interpretation, adjusted means from repeated measures analysis of covariance are included in Table S2. Error bars represent ± 1 standard error from the mean. Significance is set at + P < .10, *P < .05, ** P < .01, or *** P < .001
Diet affected t‐tau levels (diet x time F[1,58] = 4.51, P = .038); for the MCI group t‐tau was increased by the Med‐diet and decreased by the West diet (F[1,20] = 4.62, P = .044; Figure 3C). For the NC group, t‐tau was unchanged by either diet, and neither diet affected p‐tau181 for either group. A significant diet x time interaction was observed for the CSF Aβ42/t‐tau ratios (F[1,58] = 4.20, P = .045) that was moderated by a three‐way interaction with cognitive group (F[1,58] = 3.46, P = .099); the MCI group showed reduced CSF Aβ42/t‐tau ratios following the Med‐diet and increased ratios following the West‐diet (F[1,20] = 5.05, P = .036; Figure 3D), whereas ratios were unchanged for the NC group (P = .751).
3.4. Cerebral perfusion
For pcASL MRI, a comparison of cerebral perfusion for Med‐diet > West‐diet yielded clusters of significance in the left inferior frontal cortex, right middle temporal gyrus and parahippocampal gyri, posterior cingulate, precuneus, and hippocampus regions (Figure 4B). The opposite contrast (Med‐diet < West‐diet) did not yield regions of significance. Beta weights from the Med‐diet > West‐diet voxel‐wise contrast were extracted from significant clusters for both pre‐ and post‐diet pcASL MRI scans for both diets to yield an index of mean cerebral perfusion across significant voxels. A significant diet group x time interaction (F[1,49] = 4.53 P = .038) was observed for the mean perfusion index; perfusion increased following the Med‐diet and decreased following the West‐diet for the NC group (F[1,31] = 10.12 P = .003; Figure 4C). Although the MCI group perfusion values also increased following the Med‐diet, this effect was variable and did not approach significance (P = .499).
3.5. Diet effects on cognition
A three‐way interaction of diet x diagnosis x time was observed for the cognitive composite (F[1,71] = 4.00, P = .049). Although no individual comparisons between groups achieved significance, the NC group's scores tended to improve for the Med‐diet and remain unchanged for the West‐diet (F[1,45] = 3.28, P = .077), whereas the MCI group scores did not differ appreciably between diet conditions (Figure 5).
FIGURE 5.
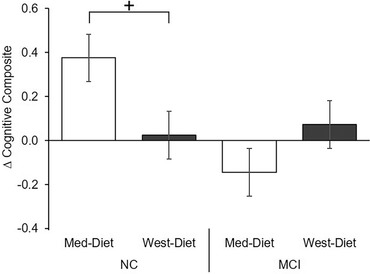
Diet effects on cognition. Diet affected cognition differently for normal cognition (NC) and mild cognitive impairment (MCI) groups (diet x diagnosis x time F[1,71] = 4.00, P = .049). No individual comparisons between groups achieved significance; the NC group's scores tended to improve for the Mediterranean diet (Med‐diet) and remain unchanged for the Western diet (West‐diet; P = .077), whereas the MCI group scores were unchanged. Figures depict change scores for ease of interpretation, adjusted means from repeated measures analysis of covariance are included in Table S2. Error bars represent ± 1 standard error from the mean. Significance is set at + P < .10, *P < .05, ** P < .01, or *** P < .001
3.6. Compliance
Compliance was excellent, with an average of <1 non‐compliant meal per week observed for each diet condition, and no differences between diagnostic groups or interactions between diet type and diagnostic groups (mean number of non‐compliant meals per week in the Med‐ and West‐diet conditions = 0.82 ± 0.24 and 0.66 ± 0.22; for the MCI group in the Med‐ and West‐diet conditions = 0.46 ± 0.40 and 0.63 ± 0.34; for the NC group = 1.19 ± .26 and 0.70 ± 0.28; all P’s n.s.). Further, the diets did not change weight for either the NC or MCI groups attesting to the accuracy of the caloric targets and participants’ compliance (all P’s n.s.).
3.7. Summary of results
A summary of diet effects is shown in Table 2. Regarding peripheral metabolism, both NC and MCI groups showed a similar response to diet for lipid levels, with the Med‐diet lowering total cholesterol, LDL, and HDL, and the West‐diet increasing lipids. Glucose and HgA1c were unaffected by the Med‐diet; levels were increased by the West‐diet for the NC group, whereas the MCI group showed lowered levels. Fasting insulin levels increased for the NC group following the Med‐diet and decreased for the MCI group following both diets. For CSF biomarkers, Aβ40 decreased after the Med‐diet and increased following the West‐diet for only the NC group. Although Aβ42 was unaffected by diet for either NC or MCI, the pattern of Aβ42/40 ratio changes differed, increasing after the Med‐diet and decreasing after the West‐diet for the NC group, with the reverse pattern demonstrated by the MCI group. Diet affected t‐tau for participants with MCI, increasing following the Med‐diet and decreasing following the West‐diet. Similarly, changes in the Aβ42/t‐tau ratio were observed for participants with MCI, with ratios lowered following the Med‐diet and increased following the West‐diet. Conversely, only NC participants showed diet effects on cerebral perfusion, which increased after the Med‐diet and decreased after the West‐diet. A trend was observed for cognition to improve for the NC group after the Med‐diet and worsen after the West‐diet.
TABLE 2.
Summary of diet effects
| Study diets | ||
|---|---|---|
| Outcomes | Med‐diet | West‐diet |
| Plasma metabolites | ||
| Total cholesterol | ↓ NC/MCI | ↑ NC/MCI |
| LDL | ↓ NC/MCI | ↑ NC/MCI |
| HDL | ↓ NC/MCI | ↑ NC/MCI |
| Glucose | No changes | ↑ NC; ↓ MCI |
| Insulin | ↑NC/↓MCI | ↓ MCI |
| HbA1c | No changes | ↑ NC; ↓ MCI |
| CSF biomarkers | ||
| Aβ40 | ↓ NC | ↑ NC |
| Aβ42 | No changes | No changes |
| Aβ42/40 | ↑ NC | ↓ NC |
| T‐tau | ↑ MCI | ↓ MCI |
| T‐tau/Aβ42 | ↓ MCI | ↑ MCI |
| P‐tau181 | No changes | No changes |
| Cerebral perfusion | ||
| Whole brain CBF | ↑ NC | ↓ NC |
| Cognition | ||
| Cognitive compositea | ↑ NC; ↓ MCI | No changes |
aOnly trend‐level effects were noted for the cognitive composite (P < .08).
Notes: Summary of plasma metabolites, CSF biomarkers, cerebral perfusion and the cognitive composite changes after the 4‐week diet intervention.
Abbreviations: Aβ, beta‐amyloid; CBF, cerebral blood flow; CSF, cerebrospinal fluid; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MCI, mild cognitive impairment; Med‐diet, Mediterranean diet; NC, normal cognition; West‐diet, Western diet.
4. DISCUSSION
This study examined the effects of a 4‐week controlled diet intervention modeled on either Western or Mediterranean dietary patterns on CSF AD biomarkers, cerebral perfusion, and cognition in middle‐aged adults who were cognitively normal or had MCI. Findings from epidemiological and basic science studies suggest dietary patterns are linked to AD risk and pathology, such that Mediterranean dietary patterns are protective, and Western patterns increase risk (as reviewed in van den Brink et al. 32 ). Much of the evidence in humans is derived from observational studies using food consumption questionnaires, particularly regarding effects of Western dietary patterns. Despite being rigorously conducted, such studies are intrinsically vulnerable to sampling biases and reverse causation. The present study used a randomized design, in which participants received foods prepared by a metabolic kitchen, ensuring their adherence to target dietary patterns. To our knowledge, this exploratory study represents the first direct comparison of Med‐ and West‐diets using a controlled intervention to determine whether diet affects indices of AD pathology and brain health in middle‐aged adults, and does so differently for CN adults and persons with MCI. As such, our results provide insight into early diet‐related mechanisms that may help to elucidate diet‐based strategies for prevention of AD as well as other causes of pathological brain aging and cognitive decline.
4.1. Diet effects on metabolism
All participants showed expected changes in blood lipid levels in response to diet, with reduced total cholesterol, LDL, and HDL following the Med‐diet and the opposite pattern observed for the West‐diet. However, the NC and MCI groups showed different glycemic responses for the West‐diet, with NC participants demonstrating increased blood glucose, insulin, and HgA1c, a pattern typically associated with an unhealthy glycemic status, and participants with MCI showing reduced levels, indicative of improved glycemic status.
4.2. Diet effects on AD biomarkers, cerebral perfusion, and cognition
We observed striking differences between NC participants and adults with MCI in diet effects on CSF AD biomarkers. For the NC adults, the Med‐diet lowered Aβ40 levels and moved the Aβ42/40 ratio in a direction associated with reduced AD risk, as well as increasing cerebral perfusion; a trend for enhanced cognition was also observed. The West‐diet increased Aβ40, lowered the Aβ42/40, ratio and reduced cerebral perfusion for NC participants, patterns associated with increased AD risk. In contrast, the overall pattern for participants with MCI was opposite to that observed for NC adults. The West‐diet raised Aβ42/40, lowered t‐tau and the Aβ42/t‐tau ratio, thus moving these indices in a direction associated with less AD pathology, whereas the Med‐diet moved them in a direction of greater pathology.
It is notable that diet modulated the Aβ42/40 ratio for both NC and MCI participants. This ratio is considered a better marker of early amyloid pathology and future development of AD than Aβ42 alone, because it corrects for intra‐individual variations in Aβ production, and/or normalizes differences in CSF dynamics related to clearance. 33 Results were consistent with prior findings that NC middle‐aged adults who adhered to a West‐like diet as assessed with a food frequency questionnaire had increased Aβ deposition on 11C Pittsburgh compound B amyloid positron emission tomography over a 3‐year period, compared to participants consuming a Med‐like diet, who showed the reverse pattern. 34
To our knowledge, this study is the first to examine the effects of a controlled diet intervention on tau in humans. T‐tau was unaffected by either diet for NC adults, which may be due in part to observations that t‐tau elevations are a later marker of neurodegeneration; 35 thus, a 4‐week diet exposure may not have been sufficient to modulate t‐tau levels in NC adults. However, for participants with MCI, both hallmarks of AD, Aβ42/40 and t‐tau, were affected by the diet intervention, consistent with studies suggesting that t‐tau changes in response to Aβ42 pathology. 36 Future studies may determine whether effects on tau are mediated through Aβ or through other pathways.
As noted, cerebral perfusion decreased for NC adults consuming the West‐diet, who showed increased Aβ40 and decreased Aβ42/40 ratios, with the opposite effects observed for the Med‐diet. This pattern is consistent with models indicating that consumption of a West‐like diet disrupts hemodynamic processes such as capillary recruitment and endothelium‐dependent vasodilation, effects that are associated with future MCI and with greater Aβ pathology. 37 These models suggest that hypoperfusion may precede and contribute to Aβ aggregation through upregulation of Aβ production, as well as through impaired Aβ clearance. 38 Thus, hypoperfusion has been proposed to be an important event early in the AD pathological cascade, most apparent in preclinical stages, and less apparent in the context of established AD pathology in MCI and AD. This possibility is consistent with our observations that cerebral perfusion was modulated by diet for NC participants, but unaffected by diet for participants with MCI, despite significantly modulating Aβ42/40 and t‐tau.
The restriction of effects on cerebral perfusion to only NC adults may also be due in part to observed diet‐related changes in Aβ40. Aβ40 is thought to have greater effects than Aβ42 on vascular integrity and function; Aβ40 deposits are a defining pathology characterizing cerebral amyloid angiopathy (CAA), a group of aging‐related brain disorders associated with microbleeds, hemorrhage, and cognitive decline. 39 The vascular preference for Aβ40, and its modulation by diet for only NC adults, may explain in part the finding of changes in cerebral perfusion for NC and not MCI. Taken together, our results suggest that diet may impact brain health through both vascular and AD‐related pathways in NC adults. The MCI participants in our study are more likely to have established AD pathology, although this possibility is difficult to verify without amyloid imaging, given the lack of data regarding established CSF AD biomarker cut‐offs in middle‐aged adults. If true, however, short‐term diet interventions may not affect vascular indices such as Aβ40 and perfusion.
4.3. Dietary modulation of peripheral lipoproteins
One pathway through which dietary intervention may have affected CSF markers of pathology and cerebral perfusion is through its effects on lipid metabolism. Lipids and lipid intermediates play fundamental roles in maintaining brain structural and functional integrity. Lipid‐derived essential fatty acids can be obtained only from the periphery, and are transported across the blood‐brain barrier (BBB) into the brain. 40 Lipid metabolism has been strongly implicated in AD pathogenesis by studies documenting the association of polymorphisms from lipid‐related genes such as APOE, CLU, ABCA7, and SORL1 with increased AD risk. 41 Western diets characterized by high intake of SF typically increase levels of cholesterol, raising LDL levels and enhancing its atherogenic potential through conformational transformation to small dense particles, as well as increasing its vulnerability to pro‐inflammatory oxidation. Conversely, Med‐like diets reduce the atherogenic and pro‐inflammatory properties of LDL. Excess LDL cholesterol results in increased oxidized cholesterol esters, which in turn may enhance the amyloidogenic pathway, and increase production of Aβ. 42 , 43 , 44 In addition to effects on Aβ production, lipid‐mediated mechanisms are integrally involved in Aβ clearance. 45 Although the interaction of lipid metabolism and tau are less studied, indirect effects via Aβ are feasible. Additionally, recent work in rodent models and in induced pluripotent stem call–derived neurons suggests that inhibiting cholesterol synthesis reduces tau pathology, and that cholesterol esters may affect tau independent of Aβ. 46 , 47 , 48
Med‐like diets have also been shown to enhance and West‐diets to reduce the function of HDL, the lipoprotein associated with risk for cardiovascular and metabolic disorders, as well as risk for AD. 49 There are a number of potential pathways through which peripheral HDL can affect central nervous system function. HDL circulating in the lumen of cerebral vessels can impact vessel health, as well as suppressing the accumulation of Aβ in the vasculature associated with CAA. 50 HDL may also reduce inflammation in cerebral vessels, and stimulate nitric oxide release from endothelial cells, enhancing vasoreactivity. Additionally, although the potential for peripheral HDL to cross the BBB is controversial, it can directly affect brain lipid metabolism through its component apolipoprotein A‐I (ApoA‐I) which is readily transported into the brain and can affect Aβ clearance. 49 HDL can also undergo direct transport via the scavenger receptor class B type 1, whose expression can be modulated by diet. 50 , 51 In the present study, peripheral HDL levels were increased by the West‐diet. Given that reduced HDL function has been reported for MCI, the West‐diet's increase in HDL availability may have offered greater benefit to adults with MCI than for NC adults. Conversely, for NC participants, enhancement of HDL function may have played a role in the Med‐diet's favorable effects on Aβ42/40 ratios and cerebral perfusion, whereas the West‐diet produced the opposite effects.
These possibilities highlight the intriguing paradox that although participants with MCI showed an identical pattern of peripheral lipid response to the diets, the direction of effects for AD biomarkers was reversed: the West‐diet was associated with a favorable AD biomarker profile for both Aβ42/40 and tau, and the Med‐diet with unfavorable changes in these measures. Although it is not possible to definitively determine the reasons for this different response pattern, it has been proposed that AD is characterized at its earliest stages by a shift away from use of glucose as a primary cerebral energy source toward fatty acid metabolism, and thus the West‐diet may have provided greater lipid‐derived bioenergetic substrates to support brain function in MCI. 52 Taken together, the improved biomarker profile in the MCI group consuming the West‐diet raises the possibility that diets that increase lipid levels and thereby provide alternative energetic substrates may benefit adults at a symptomatic stage of AD such as MCI. Conversely for NC middle‐aged adults who presumably have not experienced a bioenergetic shift, the high saturated fat West‐diet is associated with negative effects on early AD pathology and cerebrovascular health, whereas the Med‐like diet favorably alters Aβ metabolism and cerebral perfusion to preserve brain health. Notably, the pattern of results observed in this short‐term study should not be interpreted as a recommendation to increase intake of saturated fat for adults with MCI, which over longer‐term periods may have deleterious cardiovascular effects. It is likely that the apparently favorable response to increased amounts of fats for the MCI group could be obtained with diets rich in healthier mono‐ and polyunsaturated fats, although confirmation requires further study.
4.4. Limitations
Our study has several limitations. The sample size was relatively small, preventing us from directly examining important variables that may affect diet response such as sex or APOE genotype. The 4‐week diet duration was also brief, which was necessary to minimize risk to the participants randomized to the West‐diet, and thus we cannot determine counter‐regulatory or other adaptations that might occur with longer diet exposure. We also excluded participants with common medical conditions such as cardiovascular disease, hypertension, and medication‐treated hyperlipidemia to ensure safety and minimize obfuscating influences of medications, which may have resulted in an unusually healthy sample. Despite these limitations, our rigorous controlled diet intervention allowed us to see clear diet‐associated influences on key parameters of brain health.
5. CONCLUSIONS
Our results provide evidence that dietary patterns are powerful modulators of metabolic function, cerebrovascular health, AD pathology, and cognitive function. The present study provides novel evidence that for NC middle‐aged adults, adherence to a West‐like dietary pattern may confer risk for AD by perturbing metabolic health, promoting AD‐like pathology, reducing cerebral perfusion, and possibly impairing cognition, whereas a Med‐like diet promotes metabolic and brain health. Our results also raise the intriguing possibility that West‐diets may have beneficial effects for adults with MCI. These results, and identification of specific mechanisms underlying these effects, require confirmation in future larger, longer studies, but support the possibility that diet can be a powerful tool for prevention or modulation of disease progression in AD.
CONFLICTS OF INTEREST
S. Craft has received consulting fees and served as a scientific advisory board member for vTv Therapeutics, T3D Therapeutics, Cyclerion Therapeutics, and Cognito Therapeutics. Funding has been provided to her institution for research grants by NIH, the Alzheimer's Association, and Eli Lilly. A. Sanderlin had research funding provided to her institution by the Alzheimer's Association. Y. Jung had research funding provided to his institution by NIH, and holds US Patent 10,949,973 for which no payment has been received. J. Leverenz, S. Lockhart, A. Hanson, and T. Register had research funding provided to their institutions by the NIH. J. Leverenz had research funding provided to his institution by the Department of Veterans Affairs. S. Lockhart had travel funds provided to attend a conference by University of Texas Medical Branch. T. Register had travel funds provided by the Department of Defense.
Supporting information
Supporting material
Supporting material
ACKNOWLEDGMENTS
The authors would like to acknowledge the important contributions of Dr. Kaycee Sink, Patricia Wittmer, Deborah Dahl, and Linda Eastman to the conduct of the study. This work was supported by the National Institutes of Health (R37 AG‐10880; Wake Forest Alzheimer's Disease Research Center P30 AG049638; P50 NS062684; T32 AG033534) and the Department of Veterans Affairs.
Hoscheidt S, Sanderlin AH, Baker LD, et al. Mediterranean and Western diet effects on Alzheimer's disease biomarkers, cerebral perfusion, and cognition in mid‐life: A randomized trial. Alzheimer's Dement. 2022;18:457–468. 10.1002/alz.12421
REFERENCES
- 1. Dai W, Duan W, Alfaro FJ, Gavrieli A, Kourtelidis F, Novak V. The resting perfusion pattern associates with functional decline in type 2 diabetes. Neurobiol Aging. 2017;60:192‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carlsson CM. Type 2 diabetes mellitus, dyslipidemia, and Alzheimer's disease. J Alzheimer's Dis. 2010;20:711‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pelkman CL. Effects of the glycemic index of foods on serum concentrations of high‐density lipoprotein cholesterol and triglycerides. Curr Atheroscler Rep. 2001;3:456‐461. [DOI] [PubMed] [Google Scholar]
- 4. De Roos A, Van Der Grond J, Mitchell G, Westenberg J. Magnetic resonance imaging of cardiovascular function and the brain: is dementia a cardiovascular‐driven disease?. Circulation. 2017;135:2178‐2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Lowe VJ, Graff‐Radford J. Age, vascular health, and Alzheimer disease biomarkers in an elderly sample. Ann Neurol. 2017;82:706‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003;60:194‐200. [DOI] [PubMed] [Google Scholar]
- 7. Bianchi VE, Herrera PF, Laura R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr Neurosci. 2019:1‐25. [DOI] [PubMed] [Google Scholar]
- 8. Solfrizzi V, Custodero C, Lozupone M, Imbimbo BP, Valiani V, Agosti P. Relationships of dietary patterns, foods, and Micro‐ and Macronutrients with Alzheimer's disease and late‐life cognitive disorders: a systematic review. J Alzheimer's Dis. 2017;59:815‐849. [DOI] [PubMed] [Google Scholar]
- 9. Hughes TM, Craft S. The role of insulin in the vascular contributions to age‐related dementia. Biochim Biophys Acta (BBA)‐Molecular. 2016:983‐991. [DOI] [PubMed] [Google Scholar]
- 10. Yarchoan M, Toledo JB, Lee EB, Arvanitakis Z, Kazi H, Han LY. Abnormal serine phosphorylation of insulin receptor substrate 1 is associated with tau pathology in Alzheimer's disease and tauopathies. Acta Neuropathol. 2014;128:679‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodriguez‐Perdigon M, Solas M, Moreno‐Aliaga MJ, Ramirez MJ. Lipoic acid improves neuronal insulin signalling and rescues cognitive function regulating VGlut1 expression in high‐fat‐fed rats: implications for Alzheimer's disease. Biochim Biophys Acta ‐ Mol Basis Dis. 2016;1862:511‐517. [DOI] [PubMed] [Google Scholar]
- 12. Kothari V, Luo Y, Tornabene T, O'Neill AM, Greene MW, Geetha T. High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochim Biophys Acta ‐ Mol Basis Dis. 2017;1863:499‐508. [DOI] [PubMed] [Google Scholar]
- 13. Fu Z, Wu J, Nesil T, Li MD, Aylor KW, Liu Z. Long‐term high‐fat diet induces hippocampal microvascular insulin resistance and cognitive dysfunction. Am J Physiol ‐ Endocrinol Metab. 2017;312:E89‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wakabayashi T, Yamaguchi K, Matsui K, Sano T, Kubota T, Hashimoto T. Differential effects of diet‐ and genetically‐induced brain insulin resistance on amyloid pathology in a mouse model of Alzheimer's disease. Mol Neurodegener. 2019;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamamoto M, Guo D‐H, Hernandez CM, Stranahan AM. Neurobiology of disease endothelial Adora2a activation promotes blood‐brain barrier breakdown and cognitive impairment in mice with diet‐induced insulin resistance. J Neurosci. 2019;39:4179‐4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bayer‐Carter J, Green P, Montine T, VanFossen B, Baker L, Watson S. Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Arch Neurol. 2011;68:743‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanson A, Bayer‐Carter J, Green P, Montine T, Wilkinson C, Baker L. Effect of apolipoprotein E genotype and diet on apolipoprotein E lipidation and amyloid peptides: randomized clinical trial. JAMA Neurol. 2013;70:972‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hill E, Goodwill A, Gorelik A, Szoeke C. Diet and biomarkers of Alzheimer's disease: a systematic review and meta‐analysis. Elsevier Inc. 2019;76. [DOI] [PubMed] [Google Scholar]
- 19. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dement. 2011;7:270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241‐247. [DOI] [PubMed] [Google Scholar]
- 21. Teng E, Chui H. The Modified Mini‐Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314‐318. [PubMed] [Google Scholar]
- 22. Craft S, Zallen G, Baker LD. Glucose and memory in mild senile dementia of the Alzheimer Type. J Clin Exp Neuropsychol. 1992;14:253‐267. [DOI] [PubMed] [Google Scholar]
- 23. Buschke H. Selective reminding for analysis of memory and learning. J Verbal Learning Verbal Behav. 1973;12:543‐550. [Google Scholar]
- 24. Kramer J, Mungas D, Possin K, Rankin K, Boxer A, Rosen H. NIH EXAMINER: conceptualization and development of an executive function battery. J Int Neuropsychol Soc. 2014;20:19‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lim YY, Snyder PJ, Pietrzak RH, Ukiqi A, Villemagne VL, Ames D. Sensitivity of composite scores to amyloid burden in preclinical Alzheimer's disease: introducing the Z‐scores of attention, verbal fluency, and episodic memory for. Alzheimer's Dement Diagnosis, Assess Dis Monit. 2016;2:19‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jung Y, Wong EC, Liu TT. Multiphase pseudocontinuous arterial spin labeling (MP‐PCASL) for robust quantification of cerebral blood flow. Magn Reson Med. 2010;64:799‐810. [DOI] [PubMed] [Google Scholar]
- 27. Tan H, Maldjian JA, Pollock JM, Burdette JH, Yang LY, Deibler AR. A fast, effective filtering method for improving clinical pulsed arterial spin labeling MRI. J Magn Reson Imaging. 2009;29:1134‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40:383‐396. [DOI] [PubMed] [Google Scholar]
- 29. Wong EC. Quantifying CBF with pulsed ASL: technical and pulse sequence factors. J Magn Reson Imaging. 2005;22:727‐731. [DOI] [PubMed] [Google Scholar]
- 30. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage. 2003;19:1233‐1239. [DOI] [PubMed] [Google Scholar]
- 31. Wierenga CE, Hays CC, Zlatar ZZ. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer's disease. J Alzheimer's Dis. 2014;42:S411‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van den Brink A, Brouwer‐Brolsma E, Berendsen A, van de Rest O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean‐DASH Intervention for Neurodegenerative Delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer's disease‐ a review. Adv Nutr. 2019;10:1040‐1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blennow K, Zetterberg H. Biomarkers for Alzheimer's disease: current status and prospects for the future. J Intern Med. 2018;284:643‐663. [DOI] [PubMed] [Google Scholar]
- 34. Berti V, Walters M, Sterling J, Quinn CG, Logue M, Andrews R. Mediterranean diet and 3‐year Alzheimer brain biomarker changes in middle‐aged adults. Neurology. 2018;90:E1789‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hanseeuw B, Betensky R, Jacobs H, Schultz A, Sepulcre J, Becker A. Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. JAMA Neurol. 2019;76:915‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shin WS, Di J, Cao Q, Li B, Seidler PM, Murray KA. Amyloid β‐protein oligomers promote the uptake of tau fibril seeds potentiating intracellular tau aggregation. Alzheimer's Res Ther. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bangen KJ, Clark AL, Edmonds EC, Evangelista ND, Werhane ML, Thomas KR. Cerebral blood flow and amyloid‐β interact to affect memory performance in cognitively normal older adults. Front Aging Neurosci. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347‐360. [DOI] [PubMed] [Google Scholar]
- 39. Greenberg SM, Bacskai BJ, Hernandez‐Guillamon M, Pruzin J, Sperling R, van Veluw SJ. Cerebral amyloid angiopathy and Alzheimer disease — one peptide, two pathways. Nat Rev Neurol. 2020;16:30‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bruce KD, Zsombok A, Eckel RH. Lipid processing in the brain: a key regulator of systemic metabolism. Front Endocrinol (Lausanne). 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jansen I, Savage J, Watanabe K, Bryois3 J, Williams3 DM, Steinberg S. Genome‐wide meta‐analysis identifies new loci and functional pathways influencing Alzheimer's disease risk. Nat Genet. 2019;51:404‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hutter‐Paier B, Huttunen H, Puglielli L, Eckman C, Kim D, Hofmeister A. The ACAT inhibitor CP‐113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer's disease. Neuron. 2004;44:227‐238. [DOI] [PubMed] [Google Scholar]
- 43. Puglielli L, Konopka G, Pack‐Chung E, Ingano L, Berezovska O, Hyman B. Acyl‐coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid β‐peptide. Nat Cell Biol. 2001;3:905‐912. [DOI] [PubMed] [Google Scholar]
- 44. Simons M, Keller P, De Strooper B, Beyreuther K, Dotti C, Simons K. Cholesterol depletion inhibits the generation of β‐amyloid in hippocampal neurons. Proc Natl Acad Sci U S A. 1998;95:6460‐6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fan J, Donkin J, Wellington C. Greasing the wheels of Aβ clearance in Alzheimer's Disease: the role of lipids and apolipoprotein e. BioFactors. 2009;35:239‐248. [DOI] [PubMed] [Google Scholar]
- 46. van der Kant R, Goldstein LSB, Ossenkoppele R. Amyloid‐β‐independent regulators of tau pathology in Alzheimer disease. Nat Rev Neurosci. 2020;21:21‐35. [DOI] [PubMed] [Google Scholar]
- 47. Boimel M, Grigoriadis N, Lourbopoulos A, Touloumi O, Rosenmann D, Abramsky O. Statins reduce the neurofibrillary tangle burden in a mouse model of tauopathy. J Neuropathol Exp Neurol. 2009;68:314‐325. [DOI] [PubMed] [Google Scholar]
- 48. van der Kant R, Langness V, Herrera C, Williams D, Fong L, Leestemaker Y. Cholesterol metabolism is a druggable axis that independently regulates tau and amyloid‐β in iPSC‐derived Alzheimer's disease neurons. Cell Stem Cell. 2019;24:347‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Button E, Robert J, Caffrey T, Fan J, Zhao W, Wellington C. HDL from an Alzheimer's disease perspective. Curr Opin Lipidol. 2019;30:224‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vitali C, Wellington C, Calabresi L. HDL and cholesterol handling in the brain. Cardiovasc Res. 2014;103:405‐413. [DOI] [PubMed] [Google Scholar]
- 51. Fung KY, Wang C, Nyegaard S, Heit B, Fairn GD, Lee WL. SR‐BI mediated transcytosis of HDL in brain microvascular endothelial cells is independent of caveolin, clathrin, and PDZK1. Front Physiol. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Neth BJ, Craft S. Insulin resistance and Alzheimer's disease: bioenergetic linkages. Front Aging Neurosci. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting material
Supporting material


