Fig. 8.
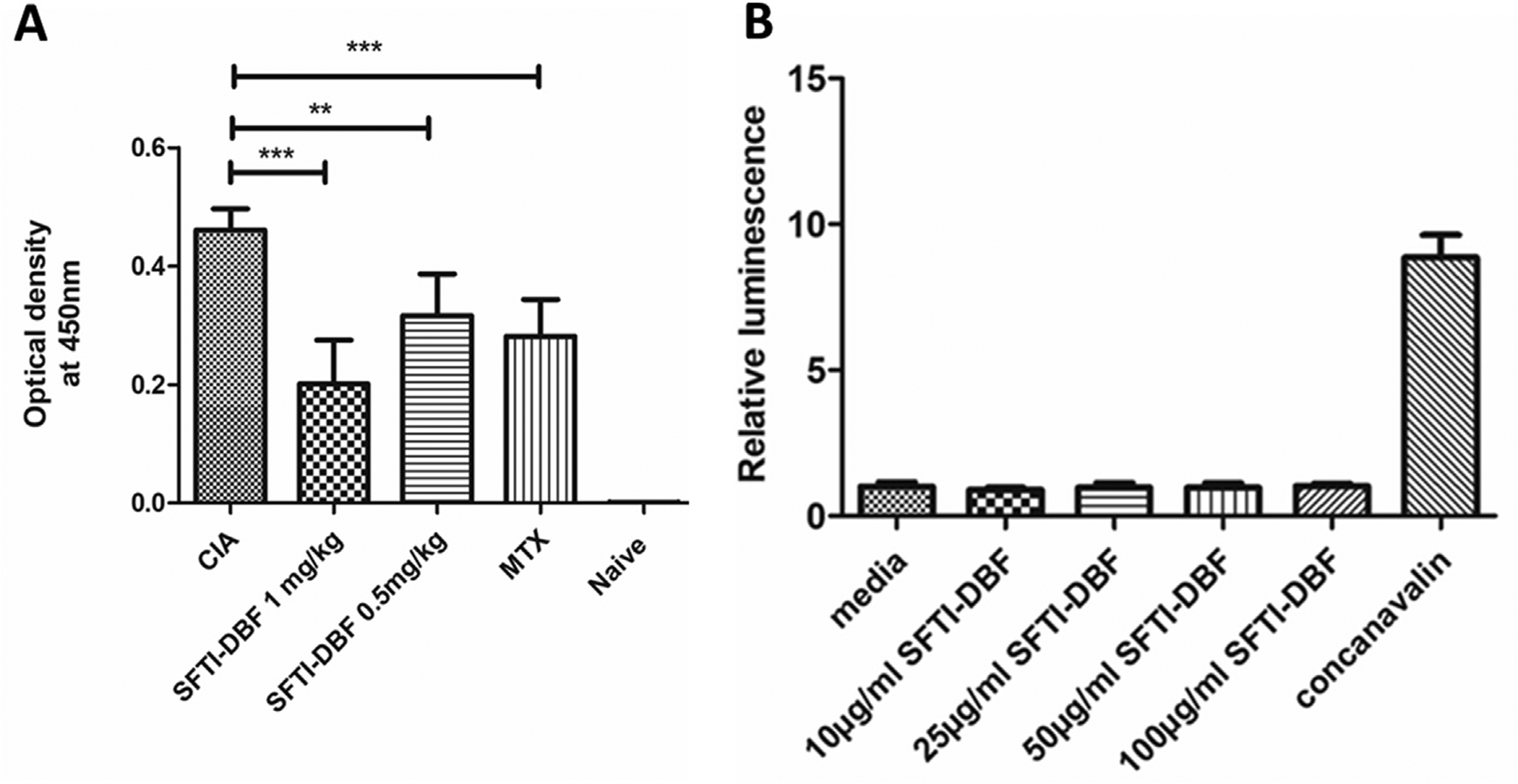
A) Reduction of circulating anti-CII Ab titer in serum of mice with CIA measured by ELISA. (N = 6). Relative levels of anti-CII Ab titer were observed with SFTI-DBF-treated mice compared to the control group (CIA). Values represent the mean ± SEM. Statistical analysis indicated that p < 0.01 (**), p < 0.001 (***) for SFTI-DBF treated mice compared to control (CIA). B) Immunogenicity study of SFTI-DBF. Splenocytes obtained from SFTI-DBF 1 mg/kg injected DBA1/J mice (N = 3) are cultured in the presence of a different concentration of SFTI-DBF (10, 25, 50, and 100 μg/mL SFTI-DBF) and T-cell mitogenic activator Concanavalin A (10 μg/mL) for 48 h. Cell proliferation is then measured by using CellTiter-Glo assay.
