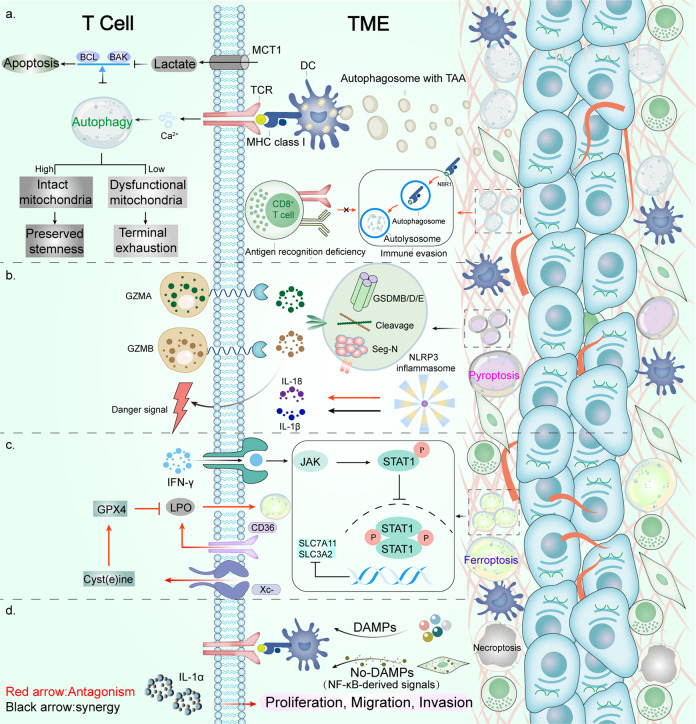
Fig. 2.
Crosstalk between T cells and dying cancer cells in the tumor microenvironment. a In dead cancer cells, autophagy increases the production of autophagosomes with TAA, which promotes DC-mediated cross-presentation. When TCR is stimulated, activated T cells have enhanced levels of autophagy, which is linked to rapidly increased calcium levels. By reprogramming metabolic pathways, autophagy is vital for mitochondrial integrity, which maintains T cells’ homeostasis. High levels of lactate in tumors inhibit autophagy and induce apoptosis in naive T cells. Furthermore, NBR1-mediated MHCI degradation through autophagy reduces MHCI expression on the surface of cancer cells and impairs CD8+ T cells recognition of antigens. b On the one hand, tumor cells via pyroptosis pathway facilitate the recruitment of CD8+ T cells by releasing danger signals. On the other hand, CD8+ T cells induce cancer cell pyroptosis by secreting GZMA and GZMB, which can cleave GSDMB/D/E. NLRP3 inflammasomes promote IL-18 and IL-1β secretion, which have tumor-promoting or antitumor effect dependent on the context of TME. c Significant lipid peroxidation activity can occur in CD36-positive CD8+ T cells, which results in ferroptosis induced by GPX4 inhibitors, leading to reduced release of IFN-γ. IFN-γ released by CD8+ T cells induces tumor cells ferroptosis through the activation of JAK1-STAT1 signaling, which transcriptionally regulates the expression of Xc- component, SLC7A11and SLC3A2. d Two strategies have been reported to trigger antitumor immunity through necroptosis. (1) DAMPs released from tumor cells through necroptosis promote cross-priming of DCs, and subsequent cytotoxic effects of CD8+ T cells. (2) Fibroblasts in the TME through necroptosis induce the robust immune response via NF-κB signaling. Besides, the necroptosis-induced release of regulatory cytokines such as IL-1α by CD8+ T cells triggers inflammation and promotes tumor growth by facilitating proliferation and migration of cancer cells