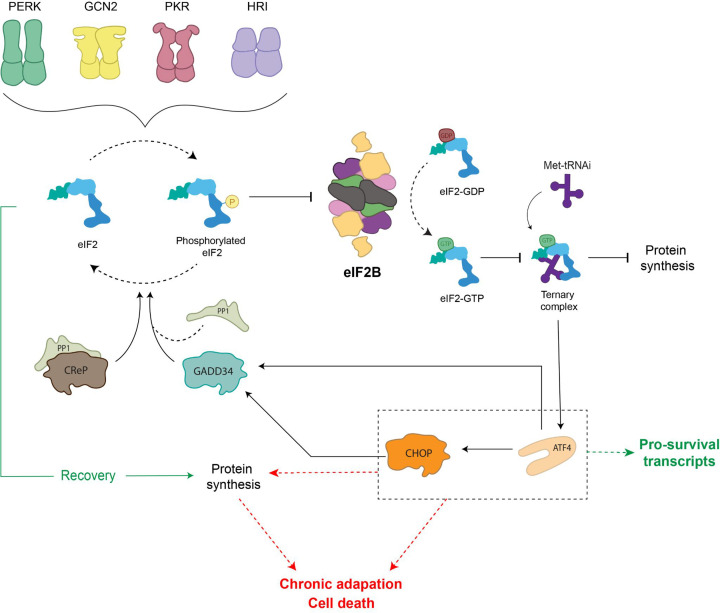
Figure 3. Activation of the ISR pathway.
In response to various cellular stress stimuli eIF2α kinase molecules are activated through dimerisation. eIF2α kinases phosphorylate the α subunit of eIF2. In its phosphorylated form, eIF2 is a competitive inhibitor of eIF2B activity preventing replenishment of eIF2-GTP within the cell. This leads to inhibition of global protein synthesis while the translation of specific stress response mRNAs, including ATF4, is up-regulated. During episodes of acute ISR, ISR effectors are able to restore homeostasis and ATF4-mediated activation of CHOP induces the transcription of GADD34 to promote dephosphorylation of eIF2α. In cases where ISR effectors are unable to restore homeostasis, the cell transitions into a chronically activated ISR. Protein synthesis is restored via an eIF2B independent mechanism and ATF4-mediated activation of CHOP promotes proapoptotic gene expression.