Fig. 5.1.
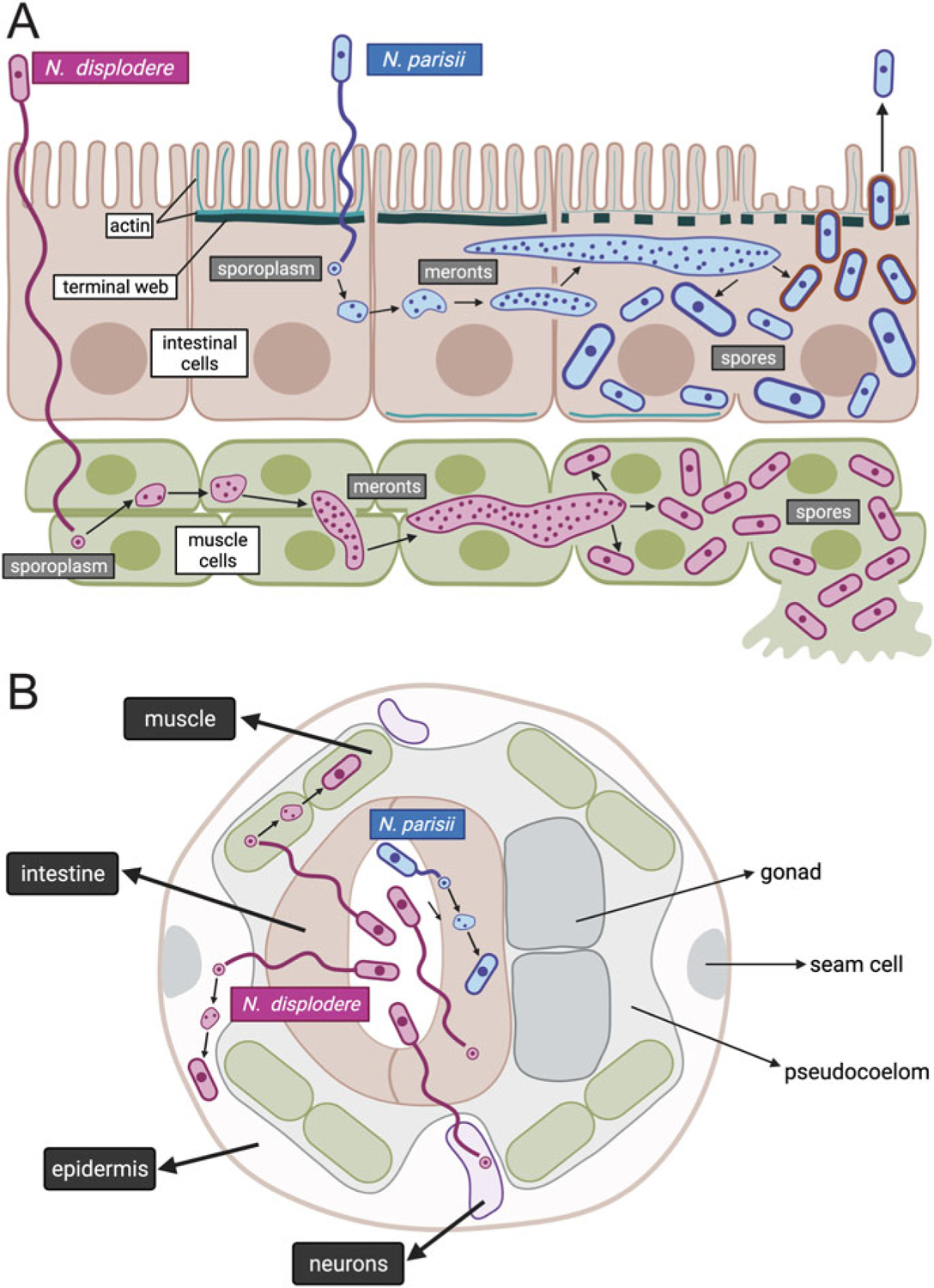
Life cycle of N. parisii and N. displodere inside the C. elegans host. Longitudinal (a) and cross-sectional (b) diagrams of Nematocida infection of the C. elegans intestine, muscle, epidermis, and neurons. N. parisii spore in blue is shown in the intestinal lumen, firing a polar tube that delivers a sporoplasm (a membrane-bound parasite cell) into the cytoplasm of C. elegans intestinal cells. This sporoplasm develops in direct contact with the host cytoplasm, replicating its nuclei without undergoing cell division, to develop into a multinucleate meront. This meront can spread across intestinal cells, causing the intestinal organ to become a syncytial structure with shared cytoplasmic contents. As N. parisii meronts develop into spores, gaps appear in the terminal web (made of actin and intermediate filaments), which is thought to remove a barrier to exit. Once N. parisii meronts develop into spores, they are found in separate membrane-bound compartments of unknown origin. These spores then become coated with the small GTPase RAB-11 (in red) and fuse with the apical membrane, to be released non-lytically back into the lumen. N. displodere spore in purple is also shown in the intestinal lumen, firing a polar tube that is longer than the polar tube of N. parisii. The N. displodere polar tube is hypothesized to reach all the way from the lumen into muscle cells, epidermal cells, and neurons to deliver sporoplasms inside of these cells. N. displodere sporoplasms develop into meronts, which can lead to fusion of muscle cells to form a syncytium (the epidermis is already a syncytium). In contrast to the non-lytic exit of N. parisii, N. displodere spores appear to burst out of cells
