Abstract
The discovery that type I interferon (IFN-α/β) inhibited tumor cell growth was welcomed initially with great excitement as it rapidly became a U.S. Food and Drug Administration–approved drug to treat several forms of cancer. In time, this enthusiasm diminished as severe toxicity associated with IFN-α administration, resistance to the therapy, or less than optimal responses became evident in cancer patients, thus restricting its clinical use and reducing its potential as an anticancer drug. The recent discovery of a third type of IFN [IFN-λ/interleukin (IL)-29/IL-28], which shares the same biological properties of type I IFNs, opens the door for evaluating the therapeutic potential of IFN-λ as it uses a distinct receptor complex whose expression, unlike type I IFN receptors, is restricted to cells of specific lineage. It is unclear whether the mechanism by which type III IFNs restrict tumor cell proliferation is different or the same from the one utilized by type I IFN. Nevertheless, accumulating evidence as described in this review suggests that, in contrast to IFN-α therapy, IFN-λ therapy could be less toxic and suitable for certain types of malignancies as not all cells are responsive to this cytokine.
Introduction
Type III interferons (IFNs), also termed IFN-λs, were discovered independently by 2 different groups in 2003 (Kotenko and others 2003; Sheppard and others 2003). The IFN-λ subfamily of IFNs consists of IFN-λ1, IFN-λ2, and IFN-λ3, also referred to as interleukin (IL)-29, IL-28A, and IL-28B, respectively. The small degree of similarity made them initially difficult to identify by sequence analysis using tools such as BLAST. The protein sequences of IFN-λs are only ∼12% identical to type I IFNs and ∼15% identical to IL-10 family members. On the basis of sequence and protein structure, IFN-λs are more similar to the IL-10 family, but the deciding factor that led to the classification of IL-29, IL-28A, and IL-28B as IFNs was their antiviral function and induction of IFN-stimulated genes (ISGs) (Fox and others 2009; Gad and others 2009).
IFN-λ signals through a heterodimeric receptor complex consisting of IFN-λ receptor 1 (IFNLR1) and interleukin-10 receptor subunit 2 (IL10R2). The IL10R2 is shared with other receptors of the IL10 receptor family and is widely expressed in different tissues (Donnelly and others 2004). In contrast, the IFNLR1 subunit has a much more restricted expression pattern. Although different in subunit composition compared with IFN-α/β, which signals through IFNAR1 and IFNAR2, the IFNLR complex triggers the activation of the same intracellular signal components, starting with JAK1 and TYK2, that can tyrosine phosphorylate STAT1, STAT2, STAT3, STAT4, and to a lesser degree STAT5, resulting in STAT dimer formation, nuclear translocation, and induction of ISG expression (Dumoutier and others 2003; Dumoutier and others 2004).
IFN-λ Responsiveness Is Restricted to Specific Cell Types
The striking similarities between type I and type III IFNs regarding their cellular effects suggested that the 2 types of IFNs had overlapping functions, leading to redundant antiviral properties. However, the difference between type I and III IFNs, apart from their structural divergence, is the cell-type and tissue-specific distribution of their respective receptor complexes. Both IFNAR1 and IFNAR2 are ubiquitously expressed, and the severe toxicity associated with IFN-α/β therapy due to unintended systemic effects has in part limited its use as a therapeutic agent (Pestka 2007). IFN-α-based treatment directly targets tumor cells or virally infected cells, as well as cells of the immune system, which exacerbate the toxicity seen in patients; therefore, the severe side effects often forces the treatment to be discontinued.
In the study by Witte and colleagues (2009), mRNA expression of IFNLR1 was evaluated in different human tissues and cell types, and their IFN-λ responses were tested. The highest mRNA expression levels were detected in lung, heart, liver, and prostate tissues, and low mRNA levels were detected in the central nervous system (CNS), bone marrow, testis, uterus, and skeletal muscles. In the skin, only keratinocytes and melanocytes showed a good correlation between receptor expression and IFN-λ-induced responses as measured by activation of STAT1 and STAT3 and upregulation of major histocompatibility complex (MHC) class I. Fibroblasts and endothelial cells were unresponsive to IFN-λ. Interestingly, naïve B and T cells express adequate amounts of IFNLR1 mRNA and yet they responded poorly or not at all to IFN-λ, respectively. The presence of a splice variant of IFNLR1 (sIFNLR1) lacking the exon coding for the transmembrane domain resulting in a frameshift producing a premature stop codon after the missing exon could account for the lack of an IFN-λ response. This truncated variant was first identified by Sheppard and coworkers (2003) and believed to be a soluble form of the receptor. The ratio of sIFNLR1 to IFNLR1 mRNA in the IFN-λ-unresponsive hematopoietic cells was higher than in responsive cells, suggesting that sIFNLR1 acts as a decoy receptor negatively regulating IFN-λ function (Witte and others 2009). This possibility was tested when expression of sIFNLR1 in HepG2 cells resulted in secretion of sIFNLR1 into the medium. sIFNLR1 could bind IFN-λ and inhibit IFN-λ signaling when preincubated before stimulation with IFN-λ. In addition, human monocytes and natural killer (NK) cells, also observed to express IFNLR1 mRNA transcripts, were found to be unresponsive to IFN-λ. This is in agreement with a recent study wherein the cytolytic activity of human NK cells was not be augmented by IFN-λ (Guenterberg and others 2010). Moreover, one contradictory finding is the observation that although IFN-λ does not induce STAT activation or MHC class I expression in monocytes, these cells and macrophages, in response to IFN-λ, can produce IL-6, IL-8, and IL-10 (Jordan and others 2007a). Similarly, human T cells were shown to respond to IFN-λ by decreasing IL-13 production and increasing IFN-γ levels when co-treated with a mitogenic stimulus (Jordan and others 2007b). A plausible explanation is that cytokine production induced by IFN-λ may be driven in a STAT-independent manner. Interestingly, human dendritic cells differentiated from monocytes appear to respond to IFN-λ and induce the proliferation of T regulatory cells (Mennechet and Uze 2006).
Compared with humans, the IFN-λ response in mice is seen in the stomach, intestines, and lungs, but is poorly induced in the CNS and spleen. However, IFN-λ promotes the proliferation of NK cells ex vivo but not in vitro and does not augment the killing activity of NK cells (Numasaki and others 2007). Further, epithelial cells of most tissues examined have been shown to express IFNLR1 mRNA transcripts. For instance, the IFN-λ response was seen in epithelial cells of the kidney and brain (Sommereyns and others 2008). These observations suggest that IFN-λ is important for protecting exposed epithelial surfaces against virus infection and subsequent spread into underlying tissues. More importantly, these studies demonstrate that cells of the immune system are not direct targets of IFN-λ, implying that severe toxicity often associated with IFN-α therapy may be absent or drastically reduced with IFN-λ.
Antiproliferative Actions of IFN-λ
IFN-α therapy continues to be used in the adjuvant treatment of certain malignancies, including melanoma, renal cell carcinoma, and chronic myeloid leukemia (Kirkwood and others 2008; McDermott 2009; Pavlovsky and others 2009). However, patients do not always complete the regimen and must discontinue treatment as they stop responding to the treatment or experience adverse effects such as fever, chills, fatigue, nausea, and joint pain caused by IFN-α administration (Pestka 2007). Numerous studies have demonstrated that type I IFNs can induce pro-apoptotic responses in primary cells, tumor cells, and immortalized cell lines (reviewed in Chawla-Sarkar and others 2003). Because signaling components are shared between type I and III IFNs, several research groups have begun to investigate the antitumor potential of IFN-λ and how this compares with IFN-α.
IFN-λ responsiveness has been documented in certain types of cancer such as neuroendocrine tumors (Zitzmann and others 2006), esophageal carcinoma (Li and others 2010), colorectal/intestinal carcinoma (Brand and others 2005), hepatocellular carcinoma (Ank and others 2006; Doyle and others 2006; Marcello and others 2006), lung adenocarcinomas (Meager and others 2005), Burkitt's lymphoma (Zhou and others 2007), and melanoma (Guenterberg and others 2010). For a list of tumor types and cell lines that are responsive to IFN-λ, please refer to Table 1. In our recent study, we demonstrated that IFN-λ1 treatment induced a prolonged but overall stronger activation of STAT1 and STAT2 in the immortalized keratinocyte line HaCaT compared with IFN-α treatment (Maher and others 2008). Another distinctive difference was the induction of ISGs by IFN-α, which peaked early and declined thereafter, whereas IFN-λ-induced ISGs levels peaked later but were sustained longer. A substantial growth inhibitory response, activation of caspase-3 and -7, and ultimately apoptosis ensued. Although IFN-α induced a modest antiproliferative effect, it did not promote apoptosis, suggesting that the prolonged STAT activity and subsequent induction and sustained expression of ISGs are what may have favored the activation of programmed cell death. Pretreatment of HaCaT cells with the pan-caspase inhibitor Z-VAD-fmk inhibited apoptosis, indicating a requirement for caspases in the promotion of IFN-λ-induced cell death. The combination of IFN-α and IFN-λ1 had an additive antiproliferative effect, suggesting that the 2 receptor complexes did not compete for available JAKs and STATs, or, alternatively, the 2 IFN types signaled partially through alternative pathways. Work by Li and colleagues (2008) has also demonstrated that IFN-λ signaling leads to the activation of apoptosis. In their model, a human HT29 colorectal adenocarcinoma cell line with ectopic expression of a chimeric IL10R1/IFNLR1 that binds IL-10, but signals through the intracellular domain of IFNLR1, was shown to undergo apoptosis when treated with IL-10. The early response was antiproliferative but later switched to one of apoptosis as a drastic increase in the proportion of sub-G0 cells was observed. Caspase-3, -8, and -9 were cleaved and activated, and pretreatment with Z-VAD-fmk abrogated caspase-3 and -9 activities, but did not block the death-inducing effect of IL-10, indicating the presence of an additional cell death pathways. Surprisingly, the Z-VAD-fmk inhibitor increased the cleavage of pro-caspase-9 into caspase-9. The strength of STAT1 activation by IL-10 through the chimeric receptor was more robust than treatment with IFN-λ that signaled through the endogenous IFNLR complex. Of note, HT29 cells express low levels of IFNLR1 and can respond to IFN-λ by upregulating MHC class I expression, but they are not growth inhibited by IFN-λ. This raises an important point as it suggests that a sufficient number of surface IFNLR1 must be expressed and engaged for IFN-λ to induce an antiproliferative effect. These studies are in agreement with our findings and strongly suggest that the strength of IFN-λ signaling through STAT activation may be the determining factor that favors an apoptotic response.
Table 1.
Human Cell Lines Responsive to Interferon-Lambda Treatment
| Tumor type | Cell line | Reference |
|---|---|---|
| Bladder carcinoma | T24/83 | Meager and others (2005) |
| Burkitt's lymphoma | Raji | Zhou and others (2007) |
| Cervical carcinoma | HeLa S3 | Kotenko and others (2003) |
| Colorectal adenocarcinoma | HT29, SW480 | Li and others (2008), Brand and others (2005), Kotenko and others (2003) |
| Colorectal carcinoma | HCT116 | Brand and others (2005) |
| Glioblastoma | LN229, LN319 | Meager and others (2005) |
| Hepatocellular carcinoma | HepG2, Huh-7 | Ank and others (2006), Marcello and others (2006) |
| Hepatoma | PEB8 | Hong and others (2007) |
| Keratinocyte carcinoma | HaCaT | Maher and others (2008) |
| Laryngeal carcinoma | Hep2C | Meager and others (2005) |
| Lung carcinoma | A549 | Kotenko and others (2003) |
| Melanoma | 1106 MEL, A375, F01 | Guenterberg and others (2010) |
| Esophageal carcinoma | T.Tn, TE-2, TE-4, TE-10, TE-12, YES-2, YES-4, YES-5 | Li and others (2010) |
| Osteosarcoma | MG63 | Meager and others (2005) |
| Pancreatic neuroendocrine carcinoma | BON1 | Zitzmann and others (2006) |
A recent study showed that in a panel of human esophageal carcinoma cell lines, the T.Tn cell line was susceptible to the apoptotic effects of IFN-λ, whereas type I IFNs elicited neither an antiproliferative nor a pro-apoptotic response (Li and others 2010). p21Waf1/Cip1 was initially highly expressed in T.Tn cells and a large fraction of these cells were in the G0/G1 phase as a possible consequence of p21 expression. In response to IFN-λ treatment, p21Waf1/Cip1 was downregulated and apoptosis was induced. In this study, however, the authors did not show a causative effect of p21 downregulation on the ensuing cell death. In fact, p21Waf1/Cip1 is known to have dual effects: it can be antiapoptotic as well as antiproliferative (reviewed in Abbas and Dutta 2009).
Responsiveness to IFN-λ is not always tumor cell-type specific. Work by Guenterberg and others screened a panel of human melanoma cell lines for IFN-λ responsiveness, and out of 8 cell lines examined, only 1 did not express IFNLR1 mRNA (Guenterberg and others 2010). Moreover, IFN-λ had no effect on inhibiting the proliferation of the melanoma cell lines tested, except for F0 melanoma cells, to which IFN-λ-induced apoptosis occurred in a dose-dependent manner. The antiproliferative and pro-apoptotic effects of IFN-λ can be augmented when used in combination with chemotherapy drugs. For instance, co-treatment of F0-melanoma cells with the proteasome inhibitor Bortezomib synergistically increased cell death, similar to the combination of IFN-α and Bortezomib (Lesinski and others 2008). The combination of 5-fluorouracil (5-FU) or cisplatin with IFN-λ also drastically inhibited cell growth of the esophageal carcinoma cell lines (Li and others 2010).
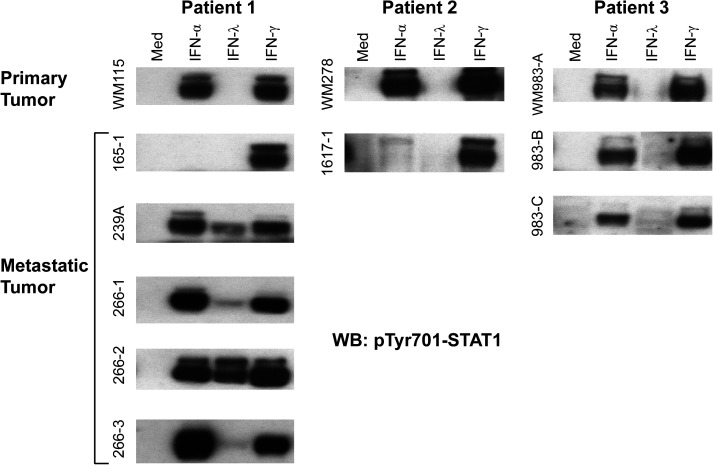
Paraffin-embedded tissue samples of primary melanoma tumors and benign nevi have been examined by in situ polymerase chain reaction and found to express transcripts for IFNLR1 (Guenterberg and others 2010). From our end we have examined paired primary and metastatic melanoma tumor cell lines established from 3 different patients. As shown in Fig. 1, all 3 primary melanoma tumors responded well to IFN-α and IFN-γ but not to IFN-λ as measured by detecting the activation of STAT1. In 1 patient, 4 out of 5 metastatic melanomas gained responsiveness to IFN-λ, suggesting the possibility that during tumor progression melanoma tumors can respond to IFN-λ. Our results also indicate that at least the primary melanoma tumor cells we examined did not respond to IFN-λ. Although we did not measure IFNLR1 mRNA expression in the established melanoma cell lines, our data imply that mRNA expression of IFNLR1 does not necessarily predict that primary melanoma tumors will respond to IFN-λ. Obviously, a large number of paired primary and metastatic melanoma tumor cell lines will be required to test this concept.
FIG. 1.
Metastatic but not primary melanoma tumor cells respond to IFN-λ. Paired primary and metastatic melanoma tumor cell lines from 3 patients were stimulated with IFN-α, IFN-λ, or IFN-γ for 20 min. IFN-λ responsiveness was measured by immunoblot analysis of phosphotyrosine-701 STAT1. IFN, interferon.
Antitumor Activity of IFN-λ
To test the antitumor potency of IFN-λ in vivo, Sato and others first transfected B16/F0 mouse melanoma cells with IFN-λ2 cDNA, which led to in vitro growth inhibition and increase in caspase-3 and caspase-7 activity (Sato and others 2006). In addition, p21Waf1/Cip1 levels increased and phosphorylation of Rb (Ser780) decreased, suggesting a mechanism for the observed cell arrest. B16/F0 cells overexpressing IFN-λ did not form pulmonary metastasis when injected into C57BL/6 mice. Histological examination of the lungs revealed cellular infiltrates and NK cells were demonstrated to be responsible for the major part of IFN-λ's antitumor effect. The effect of ectopic IFN-λ, secreted by the injected tumor cells, on the host's immune response has been thoroughly examined. In the study by Lasfar and others, mouse IFN-λ2 (mIFN-λ2)-transfected B16 melanoma cells injected subcutaneously into mice also resulted in fewer and slowly growing tumors compared with control B16 tumor cells (Lasfar and others 2006). The indirect antitumor effect of IFN-λ was confirmed by using a clone that secreted mIFN-λ2 but was unresponsive to this cytokine. IFN-λ-resistant B16 cells also grew slower in vivo and resulted in fewer tumors. Histological examination of both vector- and mIFN-λ2-secreting tumors revealed that mIFN-λ2 expression decreased the vascularization of the tumor that most likely resulted in larger areas of necrotic cell death due to hypoxia. In a different study by Numasaki and others (2007), mouse MCA205 fibrosarcoma cells were transduced with mIL-28/IFN-λ2. Unlike mIFN-λ2-secreting B16 melanoma cells, MCA205 did not respond by upregulation of MHC class I despite having receptors for IFN-λ. Subcutaneous injection of mIL-28/IFN-λ2-expressing MCA205 (MCA205IL-28) cells resulted in slowed growth of IL-28-expressing cells compared with MCA205 cells carrying an empty vector. IL-28 expressed from intravenously injected MCA205 cells also suppressed the number of developing pulmonary metastases. Moreover, nonlethal irradiation of host mice abolished the difference between MCA205IL28 cells and vector only MCA205 cells, indicating that specific immune cells are crucial for the antitumor effect of IFN-λ. Antibody depletion of targeted immune cell populations revealed that the lack of NK cells, CD8 T cells or neutrophils decreased the tumoricidal effect of IL-28/IFN-λ2. Additionally, the total number of NK cells was expanded by IL-28 and drastically increased in the presence of IL-12. It remains to be determined if the same phenomenon is also evident in human NK cells. Spleen cells from mice injected with the MCA205IL28 cells increased their IFN-γ production when re-stimulated with mIL-28/IFN-λ2 in vitro. Also, injecting MCA205IL28 cells into IFN-γ-deficient mice almost abolished the antitumor effect of mIl-28/IFN-λ2, suggesting a role for IFN-γ downstream of mIFN-λ2. In contrast to the study by Lasfar and others, tumors comprised of MCA205 cells expressing mIl-28/IFN-λ2 had an increase in infiltrating CD8 T cells.
Clinical Application of IFN-λ
The lesser antitumor effect of IFN-λ compared with IFN-α/β as seen in some model systems indicate that the effectiveness of the IFN-λs as anticancer drugs may be limiting. However, on the encouraging side, combining several anticancer drugs is a treatment strategy most often used independent of the success rate of an individual agent. So far, combining IFN-λ with more traditional anticancer agents has shown both additive and synergistic effects. Esophageal carcinoma cells treated with IFN-λ1 and 5-FU or CDDP (Cisplatin) showed an additive effect (Li and others 2010), whereas treating melanoma cells with a combination of IFN-λ1 and Bortezomib or Temozolomide had a synergistic effect (Guenterberg and others 2010).
When IFN-α was initially used for the treatment of hepatitis C and several other diseases, one major problem was its short half-life and rapid clearance from the body (Pedder 2003). This prompted the use of high concentrations of IFN-α resulting in severe acute and chronic side effects such as fever, fatigue, flu-like symptoms, hematological toxicity, and depression. Later, by conjugating polyethylene glycol moieties to IFN-α, the stability, distribution, and clearance were optimized to produce an efficient long-lasting steady state of IFN-α as well as reducing high peak concentrations and thereby decreasing some side effects. Lessons learned from the IFN-α experience has resulted in the preparation of pegylated IFN-λ1 (Peg-IFN-λ1), which is now currently in phase II clinical trials as a therapeutic option in the treatment of hepatitis C (Miller and others 2009). Thus far, animal studies and 2 previous phase I clinical studies have shown very few toxic effects in response to Peg-IFN-λ1 administration at concentrations that produce comparable antiviral effects as seen with Peg-IFN-α, measured by serum levels of β2-microglobulin, a component of MHC class I, and a marker for the antiviral effect of IFNs. The only adverse effect observed at high concentrations of Peg-IFN-λ1 was an increase of liver transaminases in serum, indicative of liver damage, which returned to normal levels after treatment ended. Overall, these results are encouraging and reveal that IFN-λ therapy may prove to be less damaging and could be used as an alternative form of therapy to IFN-α.
Enhancing the Antitumor Potency of IFN-λs
As previously mentioned, humans have 3 genes coding for IFN-λ. Since their discovery, human IFN-λ1 has been considered the most potent of the 3 in antiviral assays. In a recent study, recombinant forms of each IFN-λ were produced and their antiviral activities compared (Dellgren and others 2009). IFN-λ3 had a 2-fold higher antiviral activity compared with IFN-λ1, whereas IFN-λ2 induced a weaker response. This was surprising because IFN-λ3 shares 96% similarity with IFN-λ2. A similar comparison analysis to test the antiproliferative and antitumor potency of the IFN-λs remains to be done as this would help determine which IFN-λ is most effective to treat cancer.
One of the suggested benefits of potentially using IFN-λ in the treatment of cancer is the restricted expression of its receptor. In this condition, the decreased magnitude of IFN-λ signaling as compared with IFN-α/β is expected to cause less of the adverse side effects often seen in patients treated with type I IFNs. One strategy for increasing the antitumor efficacy of IFN-λs would be to increase the affinity of the cytokine to its receptor by using site-directed mutagenesis or phage display, with the purpose to increase or prolong the signal-inducing capacity of the ligand. A second approach would be to co-treat with a low dose of IFN-α/β or IFN-γ, which has been shown to potentiate the signals induced by IFN-λ, most likely due to increased expression of critical mediators of the JAK/STAT pathway, such as STAT1 (Li and others 2008; Maher and others 2008). A third approach would be to increase expression of the IFNLR1, preferably on tumor cells, to get more STAT activation and, consequently, increased induction of ISGs. However, it is still unknown how expression of IFNLR1 is regulated or what transcription factors are involved.
Development of an Antibody Suitable for Detection of Surface IFNLR1
During the preparation of this review, there was no antibody available that could be used for measuring cell surface expression of human IFNLR1. This, obviously, limits our ability to predict the responsiveness of tumor cells and normal cells to IFN-λ. Correlative approaches of measuring levels of IFNLR1 mRNA transcripts to predict IFN-λ responses are not always reliable indicators. Not all cell lines demonstrated to express IFNLR1 mRNA respond to IFN-λ. This alone restricts our detection tools to polymerase chain reaction and western blot analysis. The question that remains to be addressed is whether tumors that do not respond to IFN-λ are unresponsive because they lack the receptor or is it because the receptor is mutated and, therefore, is not expressed and fails to be activated by IFN-λ. Alternatively, tumor cells may secrete a truncated and soluble form of the IFNLR1 as has been shown to occur in T cells, or perhaps there may be additional regulatory components that are needed before the receptor can be activated. Several of these questions will most likely be addressed when an antibody recognizing surface IFNLR1 becomes available.
Concluding Remarks
IFN-λs are new members of the IFN family of cytokines and there is still much to learn about their mechanism of action. However, already since their discovery, their potential clinical application in treating diseases such as hepatitis C is being evaluated at a much faster pace from when type I IFNs were first identified to their introduction to the clinic. Although early to say, news that two phase 1 clinical trials show patients tolerated well IFN-λ without many of the side effects experienced with IFN-α/β is very encouraging. A phase 2 clinical trial for using Peg-IFN-λ in chronic Hepatitis C treatment is underway as of November 2009 (Miller and others 2009). If the anticipated results favor its clinical application, then the next step would be to test its antitumor efficacy in cancer patients.
Author Disclosure Statement
No potential conflicts of interest were disclosed.
References
- Abbas T. Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev. 2009;9:400–414. doi: 10.1038/nrc2657. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ank N. West H. Bartholdy C. Eriksson K. Thomsen AR. Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80:4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S. Beigel F. Olszak T. Zitzmann K. Eichhorst ST. Otte JM. Diebold J. Diepolder H. Adler B. Auernhammer CJ. Goke B. Dambacher J. IL-28A and IL-29 mediate antiproliferative and antiviral signals in intestinal epithelial cells and murine CMV infection increases colonic IL-28A expression. Am J Physiol. 2005;289:G960–G968. doi: 10.1152/ajpgi.00126.2005. . [DOI] [PubMed] [Google Scholar]
- Chawla-Sarkar M. Lindner DJ. Liu YF. Williams BR. Sen GC. Silverman RH. Borden EC. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–249. doi: 10.1023/a:1023668705040. . [DOI] [PubMed] [Google Scholar]
- Dellgren C. Gad HH. Hamming OJ. Melchjorsen J. Hartmann R. Human interferon-lambda3 is a potent member of the type III interferon family. Genes Immun. 2009;10:125–131. doi: 10.1038/gene.2008.87. . [DOI] [PubMed] [Google Scholar]
- Donnelly RP. Sheikh F. Kotenko SV. Dickensheets H. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J Leukoc Biol. 2004;76:314–321. doi: 10.1189/jlb.0204117. . [DOI] [PubMed] [Google Scholar]
- Doyle SE. Schreckhise H. Khuu-Duong K. Henderson K. Rosler R. Storey H. Yao L. Liu H. Barahmand-pour F. Sivakumar P. Chan C. Birks C. Foster D. Clegg CH. Wietzke-Braun P. Mihm S. Klucher KM. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology. 2006;44:896–906. doi: 10.1002/hep.21312. . [DOI] [PubMed] [Google Scholar]
- Dumoutier L. Lejeune D. Hor S. Fickenscher H. Renauld JC. Cloning of a new type II cytokine receptor activating signal transducer and activator of transcription (STAT)1, STAT2 and STAT3. Biochem J. 2003;370:391–396. doi: 10.1042/BJ20021935. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoutier I. Tounsi A. Michiels T. Sommereyns C. Kotenko SV. Renauld JC. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-lambda 1: similarities with type I interferon signaling. J Biol Chem. 2004;279:32269–32274. doi: 10.1074/jbc.M404789200. . [DOI] [PubMed] [Google Scholar]
- Fox BA. Sheppard PO. O'Hara PJ. The role of genomic data in the discovery, annotation and evolutionary interpretation of the interferon-lambda family. PLoS ONE. 2009;4:e4933. doi: 10.1371/journal.pone.0004933. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad HH. Dellgren C. Hamming OJ. Vends S. Paludan SR. Hartmann R. Interferon-lambda is functionally an interferon but structurally related to the interleukin-10 family. J Biol Chem. 2009;284:20869–20875. doi: 10.1074/jbc.M109.002923. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenterberg KD. Grignol VP. Raig ET. Zimmerer JM. Chan AN. Blaskovits FM. Young GS. Nuovo GJ. Mundy BL. Lesinski GB. Carson WE., 3rd. Interleukin-29 binds to melanoma cells inducing Jak-STAT signal transduction and apoptosis. Mol Cancer Ther. 2010;9:510–520. doi: 10.1158/1535-7163.MCT-09-0461. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SH. Cho O. Kim K. Shin HJ. Kotenko SV. Park S. Effect of interferon-lambda on replication of hepatitis B virus in human hepatoma cells. Virus Res. 2007;126:245–249. doi: 10.1016/j.virusres.2007.03.006. . [DOI] [PubMed] [Google Scholar]
- Jordan WJ. Eskdale J. Boniotto M. Rodia M. Kellner D. Gallagher G. Modulation of the human cytokine response by interferon lambda-1 (IFN-lambda1/IL-29) Genes Immun. 2007a;8:13–20. doi: 10.1038/sj.gene.6364348. . [DOI] [PubMed] [Google Scholar]
- Jordan WJ. Eskdale J. Srinivas S. Pekarek V. Kelner D. Rodia M. Gallagher G. Human interferon lambda-1 (IFN-lambda1/IL-29) modulates the Th1/Th2 response. Genes Immun. 2007b;8:254–261. doi: 10.1038/sj.gene.6364382. [DOI] [PubMed] [Google Scholar]
- Kirkwood JM. Tarhini AA. Panelli MC. Moschos SJ. Zarour HM. Butterfield LH. Gogas HJ. Next generation of immunotherapy for melanoma. J Clin Oncol. 2008;26:3445–3455. doi: 10.1200/JCO.2007.14.6423. . [DOI] [PubMed] [Google Scholar]
- Kotenko SV. Gallagher G. Baurin VV. Lewis-Antes A. Shen M. Shah NK. Langer JA. Sheikh F. Dickensheets H. Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. . [DOI] [PubMed] [Google Scholar]
- Lasfar A. Lewis-Antes A. Smirnov SV. Anantha S. Abushahba W. Tian B. Reuhl K. Dickensheets H. Sheikh F. Donnelly RP. Raveche E. Kotenko SV. Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma. Cancer Res. 2006;66:4468–4477. doi: 10.1158/0008-5472.CAN-05-3653. . [DOI] [PubMed] [Google Scholar]
- Lesinski GB. Raig ET. Guenterberg K. Brown L. Go MR. Shah NN. Lewis A. Quimper M. Hade E. Young G. Chaudhury AR. Ladner KJ. Guttridge DC. Bouchard P. Carson WE., 3rd. IFN-alpha and bortezomib overcome Bcl-2 and Mcl-1 overexpression in melanoma cells by stimulating the extrinsic pathway of apoptosis. Cancer Res. 2008;68:8351–8360. doi: 10.1158/0008-5472.CAN-08-0426. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. Kawamura K. Ma G. Iwata F. Numasaki M. Suzuki N. Shimada H. Tagawa M. Interferon-lambda induces G1 phase arrest or apoptosis in oesophageal carcinoma cells and produces anti-tumour effects in combination with anti-cancer agents. Eur J Cancer. 2010;46:180–190. doi: 10.1016/j.ejca.2009.10.002. . [DOI] [PubMed] [Google Scholar]
- Li W. Lewis-Antes A. Huang J. Balan M. Kotenko SV. Regulation of apoptosis by type III interferons. Cell Prolif. 2008;41:960–979. doi: 10.1111/j.1365-2184.2008.00558.x. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher SG. Sheikh F. Scarzello AJ. Romero-Weaver AL. Baker DP. Donnelly RP. Gamero AM. IFNalpha and IFNlambda differ in their antiproliferative effects and duration of JAK/STAT signaling activity. Cancer Biol Ther. 2008;7:1109–1115. doi: 10.4161/cbt.7.7.6192. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcello T. Grakoui A. Barba-Spaeth G. Machlin ES. Kotenko SV. MacDonald MR. Rice CM. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology. 2006;131:1887–1898. doi: 10.1053/j.gastro.2006.09.052. . [DOI] [PubMed] [Google Scholar]
- McDermott DF. Immunotherapy of metastatic renal cell carcinoma. Cancer. 2009;115:2298–2305. doi: 10.1002/cncr.24236. [DOI] [PubMed] [Google Scholar]
- Meager A. Visvalingam K. Dilger P. Bryan D. Wadhwa M. Biological activity of interleukins-28 and -29: comparison with type I interferons. Cytokine. 2005;31:109–118. doi: 10.1016/j.cyto.2005.04.003. . [DOI] [PubMed] [Google Scholar]
- Mennechet FJ. Uze G. Interferon-lambda-treated dendritic cells specifically induce proliferation of FOXP3-expressing suppressor T cells. Blood. 2006;107:4417–4423. doi: 10.1182/blood-2005-10-4129. . [DOI] [PubMed] [Google Scholar]
- Miller DM. Klucher KM. Freeman JA. Hausman DF. Fontana D. Williams DE. Interferon lambda as a potential new therapeutic for hepatitis C. Ann NY Acad Sci. 2009;1182:80–87. doi: 10.1111/j.1749-6632.2009.05241.x. . [DOI] [PubMed] [Google Scholar]
- Numasaki M. Tagawa M. Iwata F. Suzuki T. Nakamura A. Okada M. Iwakura Y. Aiba S. Yamaya M. IL-28 elicits antitumor responses against murine fibrosarcoma. J Immunol. 2007;178:5086–5098. doi: 10.4049/jimmunol.178.8.5086. . [DOI] [PubMed] [Google Scholar]
- Pavlovsky C. Kantarjian H. Cortes JE. First-line therapy for chronic myeloid leukemia: past, present, and future. Am J Hematol. 2009;84:287–293. doi: 10.1002/ajh.21380. . [DOI] [PubMed] [Google Scholar]
- Pedder SC. Pegylation of interferon alfa: structural and pharmacokinetic properties. Semin Liver Dis. 2003;23(Suppl 1):19–22. doi: 10.1055/s-2003-41635. . [DOI] [PubMed] [Google Scholar]
- Pestka S. The interferons: 50 years after their discovery, there is much more to learn. J Biol Chem. 2007;282:20047–20051. doi: 10.1074/jbc.R700004200. . [DOI] [PubMed] [Google Scholar]
- Sato A. Ohtsuki M. Hata M. Kobayashi E. Murakami T. Antitumor activity of IFN-lambda in murine tumor models. J Immunol. 2006;176:7686–7694. doi: 10.4049/jimmunol.176.12.7686. . [DOI] [PubMed] [Google Scholar]
- Sheppard P. Kindsvogel W. Xu W. Henderson K. Schlutsmeyer S. Whitmore TE. Kuestner R. Garrigues U. Birks C. Roraback J. Ostrander C. Dong D. Shin J. Presnell S. Fox B. Haldeman B. Cooper E. Taft D. Gilbert T. Grant FJ. Tackett M. Krivan W. McKnight G. Clegg C. Foster D. Klucher KM. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. doi: 10.1038/ni873. . [DOI] [PubMed] [Google Scholar]
- Sommereyns C. Paul S. Staeheli P. Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4:e1000017. doi: 10.1371/journal.ppat.1000017. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte K. Gruetz G. Volk HD. Looman AC. Asadullah K. Sterry W. Sabat R. Wolk K. Despite IFN-lambda receptor expression, blood immune cells, but not keratinocytes or melanocytes, have an impaired response to type III interferons: implications for therapeutic applications of these cytokines. Genes Immun. 2009;10:702–714. doi: 10.1038/gene.2009.72. . [DOI] [PubMed] [Google Scholar]
- Zhou Z. Hamming OJ. Ank N. Paludan SR. Nielsen AL. Hartmann R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J Virol. 2007;81:7749–7758. doi: 10.1128/JVI.02438-06. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzmann K. Brand S. Baehs S. Goke B. Meinecke J. Spottl G. Meyer H. Auernhammer CJ. Novel interferon-lambdas induce antiproliferative effects in neuroendocrine tumor cells. Biochem Biophys Res Commun. 2006;344:1334–1341. doi: 10.1016/j.bbrc.2006.04.043. . [DOI] [PubMed] [Google Scholar]