Abstract
Background
To limit the introduction of coronavirus disease 2019 (COVID-19) into nursing homes, restrictive measures and social distancing were implemented; however, these caused an increase in affective disorders such as depression and anxiety and an alteration of the behavioral and psychological symptoms of dementia. Therefore, it is expected that prescription trends of psychotropic drugs in nursing homes during the pandemic may have changed significantly.
Objective
This study aims to compare patterns of prescribing psychotropic drugs in nursing homes during the COVID-19 pandemic to those of the pre-pandemic period.
Methods
This cross-sectional multicenter study was conducted in geriatric units and psychogeriatric units in seven nursing homes in Gipuzkoa, Spain. On 1 March, 2020, data regarding 511 residents in geriatric units and 163 in psychogeriatric units were recorded. This study examined utilization percentages for psychotropic drugs before the pandemic (April 2018–March 2020) and during the pandemic (April 2020–March 2021) in light of projected usage based on previous years. Following the Anatomical, Therapeutic, Chemical Classification System, four therapeutic groups were analyzed: antipsychotics (N05A), benzodiazepines (N05B and N05C), antidepressants (N06A), and antiepileptic drugs (N03A).
Results
In the case of geriatric units, a downward trend of prescription was reversed for antipsychotics (−0.41; 95% confidence interval [CI] −1.41, 0.60). Benzodiazepine use also decreased less than expected (−2.00; 95% CI −3.00, −1.00). Antidepressant use increased more than predicted (0.02; 95% CI −0.97, 1.01), as did antiepileptic drug use (2.93; 95% CI 2.27, 3.60). In the psychogeriatric units, the drop in antipsychotic utilization was less than expected (−2.31; 95% CI −3.68, −0.93). Although it was expected that the prescription of benzodiazepines would decrease, usage remained roughly the same (−0.28; 95% CI −2.40, 2.34). Utilization of antidepressants (8.57; 95% CI 6.89, 10.24) and antiepileptic drugs (6.10; 95% CI 3.20, 9.00) increased significantly, which was expected, based on the forecast.
Conclusions
For all categories, usage of psychotropic drugs was higher than anticipated based on the forecast; this increase might be related to the worsening of emotional and behavioral disorders caused by the restrictive measures of the COVID-19 pandemic.
Key Points
| A high use of psychotropic drugs is observed in older adults living in nursing homes owing to the high prevalence of behavioral and psychological symptoms of dementia. |
| A relative increase in the prescription of psychotropic drugs in the pandemic period indicates a possible negative effect of emotional and behavioural disorders due to restrictive measures used to prevent infection. |
| Differences in the pattern of psychotropic drug prescription in the coronavirus disease 2019 period suggest that the effects of the pandemic are not identical in persons living in geriatric units or psychogeriatric units, thus requiring different protocols of pharmaceutical intervention. |
Introduction
When the World Health Organization declared the novel coronavirus disease 2019 (COVID-19) outbreak a pandemic on 11 March, 2020, health authorities in many countries implemented drastic lockdown measures to try to prevent the virus from spreading. Older people living in nursing homes were particularly susceptible to the coronavirus, often experiencing severe and/or lethal complications. These adverse results were related to the high prevalence of frailty and comorbidities and the cognitive deterioration, polypharmacy, and vascular changes associated with aging [1]. Additional factors contributing to these adverse results include the lack of diagnostic tests and personal protective equipment, a shortage of workers, increased social contact in shared spaces, and the urgent demand for new caregivers, many of whom needed to work in multiple institutions [2].
The spread of the infection in nursing homes was mitigated through the application of preventive and protective measures such as lockdown, isolation, avoidance of contact and proximity, mask wearing, hand washing, and social distancing. However, these measures caused an increase in affective disorders, such as depression and anxiety, as well as an alteration in the behavioral and psychological symptoms of dementia (BPSD) [3, 4]. In fact, it has been suggested that the increased use of psychotropic drugs, especially in the absence of non-pharmacological measures such as occupational therapy, memory workshops, and physical exercise, could be associated with the worsening of mental health [5, 6] in geriatric homes. A study conducted in nursing homes in the Netherlands that focused on behavioral changes between the sixth and tenth weeks following lockdowns and restrictions on visits found high levels of loneliness and depression and significant mood and behavioral changes [7].
Dementia is a highly prevalent disease that affects two thirds of institutionalized persons in nursing homes [8], 90% of them will develop at least one BPSD, and 85% will experience severe clinical implications [9]. Consequently, psychotropic drugs such as antipsychotics, antidepressants, hypnotics/anxiolytics, and antiepileptic drugs are frequently used; these drugs often pose safety issues [10]. This fact is especially important in the psychogeriatric units (PSUs) of nursing homes, where a greater use of psychotropic drugs is directly related to the clinical profile of the person admitted to these units; they tend to be younger and to have greater comorbidities of pathology and psychiatric symptomatology [11]. The present study compares prescription patterns of psychotropic medications between PSUs and geriatric units (GUs) to identify differences between both units.
The continual use of antipsychotics in people with dementia is associated with a very high mortality rate [12–15]. Although this mortality rate is influenced by a variety of factors, such as cerebrovascular and cardiovascular events, orthostatic hypotension, heart arrhythmia, prolongation of the QT interval, metabolic effects, extrapyramidal symptoms, anticholinergic effects, sedation, falls, and pneumonia, the precise mechanism causing death is not yet understood [16].
It is well known that the apathy sometimes observed in older people living in nursing homes could be both a consequence of dementia and a secondary effect of psychotropic drugs. Accordingly, the PROPER-I study conducted in nursing homes reported that only 10% of these drugs prescribed to people diagnosed with dementia were appropriate [17].
Prior to the COVID-19 pandemic, safety programs in many countries addressed the over-prescription and inappropriate use of antipsychotic and psychotropic drugs in nursing homes [18, 19]. However, data published by the National Health Service in the UK [6] from the first 3 months of the pandemic revealed an increase in the use of psychotropic drugs among people diagnosed with dementia; this increase could be related to greater anxiety and secondary psychosis due to measures aimed at preventing the virus transmission, such as isolating residents in their rooms, restricting their movements, pausing activity programs, requiring caregivers to wear personal protective equipment, and prohibiting family visits. Clearly, the geriatric care system faces new challenges in preventing severe acute respiratory syndrome coronavirus 2 transmission without resorting to the inappropriate use of psychotropic drugs to treat psycho-affective and behavioral disorders linked to the pandemic. The present study aims to analyze the patterns of prescription of antipsychotic drugs, antidepressants, hypnotic/anxiolytics, and antiepileptic drugs in the GUs and PSUs of nursing homes during the COVID-19 pandemic in comparison to anticipated patterns based on linear secular prescription trends over the last 2 years.
Methodology
Design
A cross-sectional multicenter study was conducted in the GUs (n = 511 patients) and PSUs (n = 163 patients) of seven nursing homes in Gipuzkoa (Basque Country, Spain). The nursing homes studied belong to a non-profit foundation; they each have their own physicians and standardized healthcare and social care. Older people are admitted based on the valuation scales for dependency (under the provisions of the Spanish Royal Decree 174/2011) and the Resident Assessment Instrument for Home Care (RAI-HC or interRAI-HC) [20]. Persons with significant functional and/or cognitive dependency are admitted to GUs. By contrast, persons admitted to a PSU must have a justifying diagnosis, such as a cognitive impairment or an associated neurological diagnosis, and a high-intensity behavioral disorder, such as wandering, behavioral symptoms of verbal abuse, behavioral symptoms of physical abuse, socially unacceptable/inappropriate behavioral symptoms, or refusal of care. The utilization percentages for several treatment groups were compared between the pre-pandemic (April 2018−March 2020) and pandemic (April 2020−March 2021) periods and to the values expected for the pandemic period (April 2020−March 2021) based on previous years.
Study Variables
The sample recruited on 1 March, 2020 was described to elucidate the sociodemographic and clinical profiles of people living in nursing homes. Data were collected from the electronic social and health records of the nursing homes; demographic and clinical variables measured included sex, age, length of stay in the nursing home, dementia and type of dementia, Barthel Index, Lobo’s Mini-Mental State Examination of 35 Points (the Spanish version of the Mini-Mental State Examination) [21], Reisberg’s Global Deterioration Scale [22], and the Frailty Index based on the Comprehensive Geriatric Assessment [23] (Table 1).
Table 1.
Sociodemographic and clinical characteristics of older persons in GUs and PSUs prior to the coronavirus disease 2019 pandemic
| GU, n = 511 | PSU, n = 163 | p value | |
|---|---|---|---|
| Women, n (%) | 372 (72.5) | 114 (69.9) | 0.478 |
| Average age, years (SD) | 85.75 (8.44) | 83.94 (8.93) | 0.019* |
| Median stay, months (IQR) | 22.5 (46.3) | 22.5 (37.6) | 0.410 |
| Dementia, n (%) | 238 (46.6) | 146 (89.6) | <0.001* |
| Alzheimer’s disease, n (%) | 126 (24.7) | 85 (52.1) | <0.001* |
| Vascular dementia, n (%) | 22 (4.3) | 10 (6.1) | 0.340 |
| Lewy body dementia, n (%) | 6 (1.2) | 8 (4.9) | <0.001* |
| Barthel Index, median (IQR) | 46 (62) | 34.5 (58) | 0.040* |
| Barthel Index <20, n (%) | 140 (27.5) | 52 (32.1) | 0.260 |
| MEC-35, median (IQR) | 23 (12) | 15 (11) | <0.001* |
| GDS, median (IQR) | 4 (3) | 6 (1) | <0.001* |
| GDS ≥6, n (%) | 133 (28.1) | 100 (66.2) | <0.001* |
| Frail-VIG, median (IQR) | 0.4 (0.16) | 0.48 (0.13) | <0.001* |
| Frail-VIG > 0.50, n (%) | 77 (19.6) | 46 (37.7) | <0.001* |
Frail-VIG Frailty Index based on Comprehensive Geriatric Assessment, GDS Reisberg’s Global Deterioration Scale, GU geriatric units, IQR interquartile range, MEC-35 Lobo’s Mini-Mental State Examination of 35 Points (the Spanish version of the Mini-Mental Examination), PSU psychogeriatric units, SD standard deviation, *p < 0.05
Psychotropic drug prescription in GUs and PSUs prior to the COVID-19 pandemic were also collected (Table 2). For the groups of antipsychotics and benzodiazepine (BZDs), equivalent doses were estimated in milligrams of risperidone [24] and lorazepam [25], respectively.
Table 2.
Comparison of psychotropic drug prescriptions between GUs and PSUs prior to the coronavirus disease 2019 pandemic
| GU, n = 511 | PSU, n = 163 | p value | |
|---|---|---|---|
| Drugs, median (IQR) | 7 (5) | 7 (4) | 0.580 |
| Psychotropic drugs, median (IQR) | 1 (1) | 2 (1) | < 0.001* |
| No psychotropic drugs | 114 (22.3) | 9 (5.5) | |
| 1 treatment group | 165 (32.3) | 30 (18.4) | |
| 2 treatment groups | 147 (28.8) | 62 (38.0) | |
| ≥ 3 treatment groups | 85 (16.6) | 62 (38.0) | |
| Antipsychotics | 159 (31.1) | 128 (78.5) | < 0.001* |
| Quetiapine | 83 (52.2) | 76 (59.4) | 0.224 |
| Risperidone | 39 (24.5) | 37 (28.9) | 0.403 |
| Olanzapine | 11 (6.9) | 16 (12.5) | 0.107 |
| Clozapine | 6 (3.8) | 5 (3.9) | 0.954 |
| Aripiprazole | 9 (5.7) | 1 (0.8) | 0.047* |
| Equivalent risperidone, median (IQR) | 1(2) | 1.5 (3) | 0.040 |
| Equivalent risperidone >2 mg | 48 (30.2) | 51 (39.8) | 0.100 |
| Benzodiazepines | 170 (33.3) | 47 (28.8) | 0.291 |
| Lorazepam | 96 (56.5) | 25 (53.2) | 0.689 |
| Lormetazepam | 38 (22.4) | 11 (23.4) | 0.879 |
| Diazepam | 12 (7.1) | 4 (8.5) | 0.736 |
| Alprazolam | 12 (7.1) | 2 (4.3) | 0.739 |
| Bromazepam | 8 (4.7) | 0 (0.0) | 0.206 |
| Clorazepate | 4 (2.4) | 3 (6.4) | 0.175 |
| Equivalent lorazepam, median (IQR) | 1 (1) | 1 (1) | 0.164 |
| Antidepressants | 281 (55.0) | 114 (69.9) | < 0.001* |
| Trazodone | 121 (43.1) | 55 (48.2) | 0.347 |
| SSRIs | 113 (40.2) | 46 (40.4) | 0.980 |
| Mirtazapine | 75 (26.7) | 27 (23.7) | 0.536 |
| SNRIs | 51 (18.1) | 12 (10.5) | 0.061 |
| Vortioxetine | 10 (3.6) | 2 (1.8) | 0.344 |
| Antiepileptic drugs | 110 (21.5) | 60 (36.8) | < 0.001* |
| Pregabaline | 47 (44.3) | 31 (54.4) | 0.252 |
| Levetiracetam | 25 (23.6) | 6 (10.5) | 0.043* |
| Valproic acid | 11 (10.4) | 8 (14.0) | 0.488 |
| Gabapentine | 2 (1.9) | 7 (12.3) | 0.009* |
| Topiramate | 4 (3.8) | 4 (7.0) | 0.452 |
Data are presented as n (%) unless otherwise stated
GU geriatric units, IQR interquartile range, PSU psychogeriatric units, SNRIs serotonin-norepinephrine reuptake inhibitor, SSRI selective serotonin reuptake inhibitors, *p < 0.05
The monthly proportion of people living in nursing homes with active psychotropic drug prescriptions on the first day of each month from April 2018 to March 2021 was also described. This analysis focused on the groups of antipsychotics, BZDs, antidepressants, and antiepileptic drugs. Following the Anatomical, Therapeutic, Chemical Classification System, four therapeutic groups and all the drugs included therein were analyzed: antipsychotics (N05A), BZDs (N05B and N05C), antidepressants (N06A), and antiepileptic drugs (N03A).
People living in these nursing homes who were in sociosanitary units (care programs for people with mixed social and health problems, terminal illnesses, convalescence, infectious diseases, and unresolved social problems) or physical disability units were excluded from the study. Residents who died in the first week of each month were also excluded.
Statistical Analysis
The selected variables were expressed as mean, median, and frequency (percentages). Pearson’s χ2 test was used for compare qualitative variables, while the Student’s t test and Mann−Whitney U test were used to compare the parametric and non-parametric distributions, respectively.
To calculate significant differences (p < 0.05) between the utilization percentages during the pre-pandemic and pandemic periods and between the pre-pandemic period and the expected percentages for the pandemic period based on secular trend lines, Pearson’s χ2 test was used. Statistical analysis was performed using SPSS software (SPSS Inc., Chicago, IL, USA, version 20.0).
Results
The sociodemographic and clinical characteristics for people living in the GUs and the PSUs of nursing homes on 1 March, 2020 were analyzed separately, and the two groups were compared (Table 1). The study population was 674 residents, with an average age of 85.3 years and a median stay of 22.5 months. The PSU residents were younger on average and showed a higher prevalence for dementia (46.6% vs 89.6%), more advanced frailty (Frailty Index based on the Comprehensive Geriatric Assessment >0.5: 19.6% vs 37.7%), and greater functional deterioration (median Barthel Index: 46 [interquartile range (IQR) 62] vs 34.5 [IQR 58]) and cognitive deterioration (median Reisberg’s Global Deterioration Scale: 4 [IQR 3] vs 6 [IQR 1]).
In the analyzed sample, the median number of drug prescriptions was seven, with no differences between the two care units. However, while the use of psychotropic drugs was predominantly greater in the PSUs, no change was observed in BZD use in GUs (Table 2). Among the antipsychotics, the most frequently prescribed active ingredient was quetiapine, followed by risperidone and olanzapine. Residents with an active prescription for antipsychotics consumed the equivalent of a median of 1.34 mg of risperidone, with the largest doses used in the PSUs (1 mg [IQR 2] in GUs vs 1.5 mg [IQR 3] in PSUs, p < 0.05). Regarding the BZD group, lorazepam and lormetazepam were used, with no difference between the GUs and the PSUs in the frequency of use or size of dose. The most-used antidepressants were trazodone and selective serotonin reuptake inhibitors, more than mirtazapine and serotonin-norepinephrine reuptake inhibitors; selective serotonin reuptake inhibitors were used more in the PSUs. Antiepileptic drugs were also used more in the PSUs than the GUs. In the GUs, use of levetiracetam increased, while use of gabapentin decreased.
During the pre-pandemic period, there was an average of 513 residents in the GUs, during the pandemic period, the average dropped to 475 residents (average difference 38.33; 95% CI 25.51, 51.16; p < 0.01). In the PSUs, there was an average of 158 residents during the pre-pandemic period, with no significant change during the pandemic period, when the average was 155 residents (average difference 2.83; 95% CI −0.32, 5.99; p = 0.237).
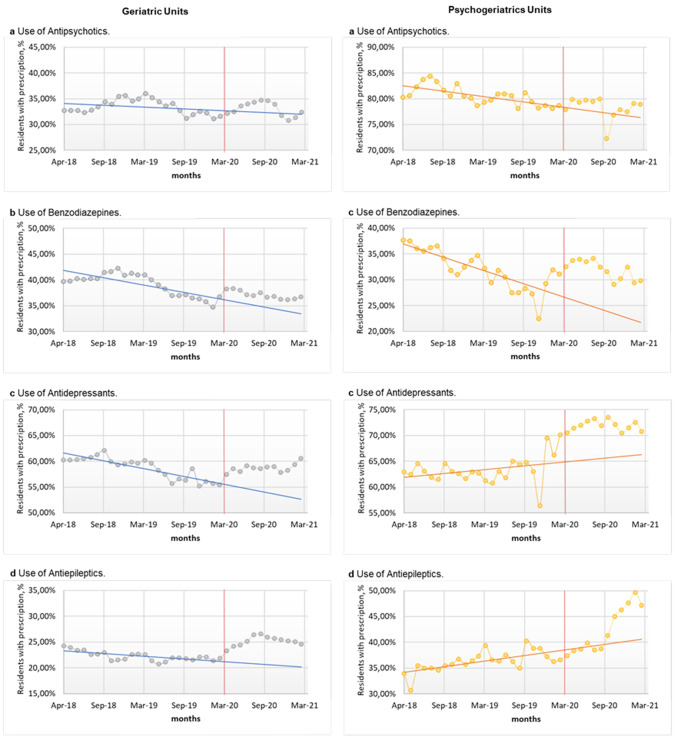
In the GUs, antipsychotic consumption remained stable during the pandemic compared to the pre-pandemic period (−0.41, 95% CI −1.41, −0.60; p = 0.416) (Table 3), contrary to the forecasts that predicted a decrease (Fig. 1). In the PSUs, by contrast, the average consumption of antipsychotics decreased (−2.31; 95% CI −3.63, − 0.93; p < 0.001), although by less than forecasted.
Table 3.
Comparative analysis of the prescription of psychotropic drugs in GUs and PSUs during the pre-pandemic and pandemic periods
| Treatment group | Pre-pandemic (April 2018−March 2020) | Pandemic (April 2020−March 2021) | Trend (April 2020−March 2021) | ||||
|---|---|---|---|---|---|---|---|
| Monthly averagea, % |
Monthly averagea, % | Differencec, % (95% CI) |
p value | Monthly averageb, % | Differenced, % (95% CI) |
p value | |
| Antipsychotics | |||||||
| GU | 33.43 | 33.01 | −0.41 (−1.41, 0.60) | 0.416 | 31.21 | −2.22 (−3.15, −1.28) | <0.001 |
| PSU | 80.53 | 78.23 | −2.31 (−3.68, −0.93) | <0.001 | 77.05 | −3.48 (−4.32, −2.64) | <0.001 |
| Benzodiazepines | |||||||
| GU | 39.10 | 37.10 | −2.00 (−3.00, −1.00) | <0.001 | 33.61 | −5.49 (−6.92, −4.06) | <0.001 |
| PSU | 31.95 | 31.93 | −0.28 (−2.40, 2.34) | 0.981 | 24.62 | −7.33 (−9.08, −5.58) | <0.001 |
| Antidepressants | |||||||
| GU | 58.70 | 58.72 | 0.02 (−0.97, 1.01) | 0.962 | 53.47 | −5.23 (−6.37, −4.09) | <0.001 |
| PSU | 63.35 | 71.92 | 8.57 (6.89, 10.24) | <0.001 | 66.52 | 3.17 (1.49, 4.86) | <0.001 |
| Antiepileptic drugs | |||||||
| GU | 22.23 | 25.16 | 2.93 (2.27, 3.60) | <0.001 | 21.06 | −1.17 (−1.56, −0.78) | <0.001 |
| PSU | 36.29 | 42.39 | 6.10 (3.20, 9.00) | <0.001 | 38.89 | 2.60 (1.76, 3.44) | <0.001 |
GU geriatric unit, PSU psychogeriatric unit
aMonthly average of residents with active prescriptions from the different treatment sub-groups studied. On 1 March, 2020, 674 residents were recorded in the seven studied nursing homes, with 511 in the GUs and 163 in the PSUs
bMonthly average predicted for the different treatment sub-groups based on the prior usage proportions between April 2018 and March 2020
cAbsolute differences between the pandemic period (April 2020–March 2021) vs the pre-pandemic period (April 2018–March 2020)
dAbsolute differences between the prescription proportions forecast for the pandemic period (April 2020–March 2021) vs the pre-pandemic period (April 2018–March 2020)
Fig. 1.
Changes in the prescription of psychotropic drugs in nursing homes from April 2018 to March 2021. Representation by the treatment sub-groups of the monthly proportion of residents taking some psychotropic drug from April 2018 to March 2021, in the different residential units (geriatric unit, psychogeriatric unit). The vertical dotted red line represents the date that coronavirus disease 2019 appeared in the Basque Country (March 2020). The secular trend lines are based on consumption proportions from April 2018 to March 2020
The average use of BZDs during the pandemic period decreased in the GUs (−2.00; 95% CI −3.00, −1.00; p < 0.001), although by less than anticipated based on the linear secular trends. In the PSUs, the use of BZDs during the pandemic period showed no change (−0.28; 95% CI −2.40, 2.34; p = 0.981) compared to the pre-pandemic period, even though a considerable decrease in its use was expected.
The use of antidepressants remained constant (0.02; 95% CI −0.97, 1.01; p = 0.962) during the pandemic period in the GUs, while the trends predicted a decrease in their use. In the PSUs, the use of antidepressants increased more than anticipated (8.57; 95% CI 6.89, 10.24; p < 0.001). Finally, the use of antiepileptic drugs increased significantly both in the GUs (2.93; 95% CI 2.27, 3.60; p < 0.001) and the PSUs (6.10; 95% CI 3.20, 9.00; p < 0.001), while the trends predicted a slight decrease in the use of GUs and a slighter increase in the use of PSUs.
Discussion
Research has shown that the utilization of psychotropic drugs has increased in the geriatric population in nursing homes, due, in part, to off-label use [26–28]. However, PSUs and GUs in nursing homes show a differential prescription pattern, as sociodemographic characteristics, cognitive and frailty factors, and psychotropic drug utilization differ between the units. This observation agrees with the previously cited studies by Saarela et al. [11] and Tordoff et al. [29]. The findings reported in this paper demonstrate, for the first time, that prescription dynamics for psychotropic drugs during the pandemic differed in PSUs and GUs, indicating a differential profile that could be important when designing specific protocols for caring for and addressing BPSD.
Although an appropriate selection of antipsychotics was used to control BPSD, with a predominance of atypical drugs both in GUs and PSUs, more than a third of patients treated with antipsychotics exceeded the daily doses equivalent to 2 mg per day of risperidone, the maximum dose recommended on the technical data sheet. The presence of patients with psychiatric pathologies, such as schizophrenia or bipolar disorder, leads to higher dosages, sometimes beyond the recommended dosage, which generates a greater risk of adverse events such as hospitalization, falls and fractures, and mortality, with a dose–response relationship [30–33].
Up to one third of the study population consumed some type of BZD, mainly as a hypnotic drug and to control anxiety. Lorazepam, the most used drug, has a greater safety profile than others in the same class because of its resistance to oxidative metabolism and a lesser predisposition to accumulate in the body [34]. However, regardless of the half-life and hepatic metabolism of the drug, older adults have a higher sensitivity to BZDs; in general, all BZDs increase the risk of cognitive impairment, delirium, falls, and fractures [35].
Antidepressants were the most-used group of psychotropic drugs, with 58.6% of persons taking this treatment. Among the drugs in this group, trazodone, a drug that is mainly associated with an indication of insomnia in patients with a depressive syndrome, was used most frequently; mirtazapine was used similarly. Selective serotonin reuptake inhibitors were the second most-used class of antidepressants, in this case for an indication of depressive syndrome, followed by serotonin-norepinephrine reuptake inhibitors, then tricyclic antidepressants.
This study found that the proportions of psychotropic drug utilization were, in all cases, greater than expected based on secular prescription trends. This findings agrees with the results of a cross-sectional study conducted on 77,291 residents living in 623 licensed nursing homes in Ontario (Canada) [5], which found an absolute increase in the use of antipsychotics, antidepressants, and trazodone between March and September 2020 and between January and February 2020 in nursing homes during the COVID-19 pandemic. A decrease in the use of BZDs was observed, contrary to the expectation based on secular trends that a slight increase in use would occur.
Moreover, a study conducted in the UK [6], based on data from the English National Health Service, analyzed the use of antipsychotics in people diagnosed with dementia. Howard et al. reported an increase in the use of antipsychotics in patients with dementia during the first period of the pandemic (March−May 2020), compared with the pre-pandemic period.
There was an increased use of antiepileptic drugs, which are sometimes prescribed as mood stabilizers to control BPSD. Trends for the pandemic period predicted an increase in the use of these drugs, mainly in the PSUs, perhaps filling the gap left by other groups of drugs that were decreasing in usage, such as antipsychotics and BZDs. Some studies of nursing homes in the USA [36, 37] and Canada [38] also observed this new prescription practice, particularly following the US Food and Drug Administration’s boxed warning [39] describing the side effects to the use of antipsychotics.
In some cases, however, lessened effectivity and many related side effects have been reported for these alternative mood stabilizers [40]. Thus, valproic acid and carbamazepine have not been suggested as effective alternatives for controlling BPSD, as both have severe adverse effects [41]. In fact, many initiatives encouraging the decreased use of antipsychotics have led to the prescription of other psychotropic drugs, which can be summarized as the same way of operating but with a different name.
This increase in the use of psychotropic drugs in nursing homes, possibly caused by an increase in secondary behavioral disorders and mood swings due to the pandemic, represents a deceleration in the suitability of psychotropic drugs in nursing homes. Although guides for BPSD treatment from the International Psychogeriatric Association [42] clearly recommend the first-line implementation of non-pharmacological strategies, it has been difficult to apply these strategies in institutions where staff are extremely overworked because of COVID-19.
It is essential, therefore, to strengthen the incentives for training programs for health workers and caregivers, such as the describe, investigate, create, evaluate (DICE) focus [43], OASIS [44], or chemical restraint avoidance methodology (CHROME) criteria [19], which propose new conceptual models to evaluate and respond to contributing factors for BPSD. Specifically, CHROME, published by a panel of experts in Spain, has two objectives: (1) offer a method for the elimination of chemical restraints in institutionalized people with dementia, with verifiable and auditable results and (2) contribute to improving the quality of life of people with dementia in geriatric institutions through the rational and safe use of psychotropic drugs, within a legal framework. Compared to simply prescribing drugs, these models require a reorganization of dementia care to give prescribing physicians and health professionals more time to explain and address the modifiable triggers caused by BPSD.
Implementing these models will increase the use of non-pharmacological measures, reserving pharmacological measures for severe situations based on proven scientific evidence. Thus, it is essential to set up multidisciplinary teams composed of caregivers, family members, psychologists, psychiatrists, physicians, and pharmacists to define care goals together and reassess and monitor the benefit–risk ratio of interventions.
A limitation of this study is that, for the GUs, the suspension of new admissions to nursing homes and deaths due to COVID-19 reduced the number of residents during the pandemic period, making it more difficult to interpret the tendency toward using psychotropic drugs among these residents. Although sociosanitary variables in these residents could have changed because of the pandemic, their sociodemographic data were not collected during the second period of this study. However, in the PSUs, the resident censuses did not vary significantly. Another limitation of this study is that it did not capture the indication for which the psychotropic drug was prescribed nor the duration of treatment, making it difficult to elucidate the degree of suitability of the prescriptions
Considering the increase in psychotropic drug utilization during the pandemic period and the possible worsening of BPSD in persons with dementia, it is necessary to apply adequate pharmacological measures and implement complementary non-pharmacological measures. Thus, to clearly differentiate the protocols used in PSUs from those used for ordinary residents of nursing homes, it is essential to develop a new therapeutic strategy that avoids a systematic increase in the use of psychotropic drugs, such as the creation of new care protocols and monitoring in persons with dementia whose BPSD is worsening under lockdowns and isolation.
Conclusions
The results of this study show a greater use of psychotropic drugs compared with the forecast, this increase might be related to the worsening of emotional and behavioral disorders in people living in nursing homes due to isolation during the COVID-19 pandemic, and the consequent absence of non-pharmacological measures and lack of systematic control of pharmacological therapy.
Acknowledgements
The authors express their gratitude to Olatz Hijarrubia Lanau (Pharmacy Degree Student, University of the Basque Country) for her collaboration in the data collection of this research.
Declarations
Funding
No sources of funding were received for the preparation of this article or the condut of this study.
Conflicts of interest/competing interests
Alexander Ferro Uriguen, Esther Laso Lucas, Cinzia Sannino Menicucci, Izaskun Iturrioz Arrechea, Javier Alaba Trueba, Enrique Echevarría Orella, Javier Gil Goikouria, and Idoia Beobide Telleria have no conflicts of interest that are directly relevant to the content of this study.
Ethics approval
The study was approved (approval number: EOM2021016) for execution by the Euskadi Drug Research Ethics Committee in Vitoria-Gasteiz (Basque Country, Spain).
Consent to participate
Given the characteristics of the study, the Euskadi Drug Research Ethics Committee exempted the need for informed consent. User identification data were anonymized in the database.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
AFU formulated the research question. AFU, ELL, CSM, IIA, and IBT contributed to the conception and design of the work. AFU, IBT, EEO, and JGG analyzed the data. The first draft of the manuscript was written by AFU. AFU, ELL, CSM, IIA, JAT, EEO, JGG, and IBT critically reviewed the manuscript. All authors approved the final version for publication.
References
- 1.Inzitari M, Risco E, Cesari M, Buurman BM, Kuluski K, Davey V, et al. Nursing homes and long term care after COVID-19: a new era? J Nutr Health Aging. 2020;24:1042–1046. doi: 10.1007/s12603-020-1447-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ministry of Social Rights and the 2030 Agenda. Report of the Working Group on COVID-19 and Residences. 2020. https://www.euskadi.eus/gobierno-vasco/contenidos/documentacion/doc_sosa_gt_covid_residencias/es_def/index.shtml. Accessed 20 Dec 2021.
- 3.Abbasi J. Social isolation: the other COVID-19 threat in nursing homes. JAMA. 2020;324:619–620. doi: 10.1001/jama.2020.13484. [DOI] [PubMed] [Google Scholar]
- 4.Palmer K, Monaco A, Kivipelto M, Onder G, Maggi S, Michel JP, et al. The potential long-term impact of the COVID-19 outbreak on patients with non-communicable diseases in Europe: consequences for healthy ageing. Aging Clin Exp Res. 2020;32:1189–1194. doi: 10.1007/s40520-020-01601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stall NM, Zipursky JS, Rangrej J, Jones A, Costa AP, Hillmer MP, et al. Assessment of psychotropic drug prescribing among nursing home residents in Ontario, Canada, during the COVID-19 pandemic. JAMA Intern Med. 2021;181:861–863. doi: 10.1001/jamainternmed.2021.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard R, Burns A, Schneider L. Antipsychotic prescribing to people with dementia during COVID-19. Lancet Neurol. 2020;19:892. doi: 10.1016/S1474-4422(20)30370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Roest HG, Prins M, Van der Velden C, Steinmetz S, Stolte E, de Vries DH. The impact of COVID-19 measures on well-being of older long-term care facility residents in the Netherlands. J Am Med Dir Assoc. 2020;21:1569–1570. doi: 10.1016/j.jamda.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López Mongil R, López Trigo JA, Castrodeza Sanz FJ, Tamames Gómez S, León Colombo T, Muñoz Rivero T, et al. Prevalence of dementia in institutionalized patients. The RESYDEM study. Rev Esp Geriatr Gerontol. 2009;44:5–11. doi: 10.1016/j.regg.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Lyketsos CG. Neuropsychiatric symptoms (behavioral and psychological symptoms of dementia) and the development of dementia treatments. Int Psychogeriatr. 2007;19:409–420. doi: 10.1017/S104161020700484X. [DOI] [PubMed] [Google Scholar]
- 10.Magierski R, Sobow T, Schwertner E, Religa D. Pharmacotherapy of behavioral and psychological symptoms of dementia: state of the art and future progress. Front Pharmacol. 2020;11:1–15. doi: 10.3389/fphar.2020.01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saarela TM, Finne-Soveri H, Liedenpohja AM, Noro A. Comparing psychogeriatric units to ordinary long-term care units: are there differences in case-mix or clinical symptoms? Nord J Psychiatry. 2008;62:32–38. doi: 10.1080/08039480801960172. [DOI] [PubMed] [Google Scholar]
- 12.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. J Am Med Assoc. 2005;294:1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 13.Ma H, Huang Y, Cong Z, Wang Y, Jiang W, Gao S, et al. The efficacy and safety of atypical antipsychotics for the treatment of dementia: a meta-analysis of randomized placebo-controlled trials. J Alzheimers Dis. 2014;42:915–937. doi: 10.3233/JAD-140579. [DOI] [PubMed] [Google Scholar]
- 14.Kales HC, Kim HM, Zivin K, Valenstein M, Seyfried LS, Chiang C, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169:71–79. doi: 10.1176/appi.ajp.2011.11030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ralph SJ, Espinet AJ. Increased all-cause mortality by antipsychotic drugs: updated review and meta-analysis in dementia and general mental health care. J Alzheimers Dis Rep. 2018;2:1–26. doi: 10.3233/ADR-170042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinberg M, Lyketsos CG. Atypical antipsychotic use in patients with dementia: managing safety concerns. Am J Psychiatry. 2012;169:900–906. doi: 10.1176/appi.ajp.2012.12030342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Der Spek K, Gerritsen DL, Smalbrugge M, Nelissen-vrancken MHJMG, Wetzels RB, Smeets CHW, et al. Only 10 % of the psychotropic drug use for neuropsychiatric symptoms in patients with dementia is fully appropriate. The PROPER I-study. Int Psychogeriatr. 2016;28:1589–1595. doi: 10.1017/S104161021600082X. [DOI] [PubMed] [Google Scholar]
- 18.Westbury JL, Gee P, Ling T, Brown DT, Franks KH, Bindoff I, et al. RedUSe: reducing antipsychotic and benzodiazepine prescribing in residential aged care facilities. Med J Aust. 2018;208:398–403. doi: 10.5694/mja17.00857. [DOI] [PubMed] [Google Scholar]
- 19.Olazarán-Rodríguez J, López-álvarez J, Agüera-ortiz LF, López-arrieta JM, Beltrán-aguirre JL, García-garcía P, et al. Criterios CHROME para la acreditación de centros libres de sujeciones químicas y para una prescripción de psicofármacos de calidad. Psicogeriatría. 2016;6:91–98. [Google Scholar]
- 20.interRAI. Improving health care across the globe. https://interrai.org/. Accessed 27 Apr 2022.
- 21.Lobo A, Ezquerra J, Gómez Burgada F, Sala JM, Seva DA. El miniexamen, cognoscitivo (un “test” sencillo, práctico, para detectar alteraciones intelectuales en pacientes médicos) Actas Luso Esp Neurol Psiquiatr Cienc Afines. 1979;7:189–202. [PubMed] [Google Scholar]
- 22.Reisberg B, Ferris S, De Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 23.Amblàs-Novellas J, Martori JC, Molist Brunet N, Oller R, Gómez-Batiste X, Espaulella PJ. Frail-VIG index: design and evaluation of a new frailty index based on the Comprehensive Geriatric Assessment. Rev Esp Geriatr Gerontol. 2017;52:119–127. doi: 10.1016/j.regg.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Woods S. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/JCP.v64n0607. [DOI] [PubMed] [Google Scholar]
- 25.Sussex Partnership NHS. Calculating equivalent doses of oral benzodiazepines Background. 2017. http://www.sussexpartnership.nhs.uk/sites/default/files/documents/bdz_equivalent_doses_spt_guidance_update_-_0714.pdf. Accessed 27 Sep 2021.
- 26.Janus SIM, Van Manen JG, Ijzerman MJ, Zuidema SU. Psychotropic drug prescriptions in Western European nursing homes. Int Psychogeriatr. 2016;28:1775–1790. doi: 10.1017/S1041610216001150. [DOI] [PubMed] [Google Scholar]
- 27.Jester DJ, Molinari V, Zgibor JC, Volicer L. Prevalence of psychotropic polypharmacy in nursing home residents with dementia: a meta-analysis. Int Psychogeriatr. 2021;33:1083–1098. doi: 10.1017/S1041610220004032. [DOI] [PubMed] [Google Scholar]
- 28.French DD, Campbell RR, Spehar AM, Accomando J. How well do psychotropic medications match mental health diagnoses? A national view of potential off-label prescribing in VHA nursing homes. Age Ageing. 2007;36:107–112. doi: 10.1093/ageing/afl131. [DOI] [PubMed] [Google Scholar]
- 29.Tordoff JM, Ailabouni NJ, Browne DP, Al-Sallami HS, Gray AR. Improvements in the prescribing of antipsychotics in dementia and psychogeriatric units in New Zealand. Int J Clin Pharm. 2016;38:941–949. doi: 10.1007/s11096-016-0318-1. [DOI] [PubMed] [Google Scholar]
- 30.Lapeyre-Mestre M. A review of adverse outcomes associated with psychoactive drug use in nursing home residents with dementia. Drugs Aging. 2016;33:865–888. doi: 10.1007/s40266-016-0414-x. [DOI] [PubMed] [Google Scholar]
- 31.Huybrechts KF, Gerhard T, Crystal S, Olfson M, Avorn J, Levin R, et al. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: population based cohort study. BMJ. 2012;344:e977. doi: 10.1136/bmj.e977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maust DT, Kim HM, Seyfried LS, Chiang C, Kavanagh J, Schneider LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiat. 2015;72:438–445. doi: 10.1001/jamapsychiatry.2014.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simoni-Wastila L, Wei YJ, Lucas JA, Brandt N, Moyo P, Huang TYJ, et al. Mortality risk of antipsychotic dose and duration in nursing home residents with chronic or acute indications. J Am Geriatr Soc. 2016;64:973–980. doi: 10.1111/jgs.14111. [DOI] [PubMed] [Google Scholar]
- 34.Velert Vila J, Velert Vila MDM, Salar Ibáñez L, Avellana Zaragoza JA, Moreno RL. Suitability of the use of benzodiazepines prescribed by the pharmacist in the elderly: a doctor-pharmacist collaboration study. Aten Primaria. 2012;44:402–410. doi: 10.1016/j.aprim.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67:674–694. doi: 10.1111/jgs.15767. [DOI] [PubMed] [Google Scholar]
- 36.Maust DT, Kim HM, Chiang C, Kales HC. Association of the centers for medicare & medicaid services’ national partnership to improve dementia care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med. 2018;178:640–647. doi: 10.1001/jamainternmed.2018.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kales HC, Zivin K, Kim HM, Valenstein M, Chiang C, Ignacio R, et al. Trends in antipsychotic use in dementia 1999–2007. Arch Gen Psychiatry. 2011;68:190–197. doi: 10.1001/archgenpsychiatry.2010.200. [DOI] [PubMed] [Google Scholar]
- 38.Vasudev A, Shariff SZ, Liu K, Burhan AM, Herrmann N, Leonard S, et al. Trends in psychotropic dispensing among older adults with dementia living in long-term care facilities: 2004–2013. Am J Geriatr Psychiatry. 2015;23:1259–1269. doi: 10.1016/j.jagp.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 39.FDA News. FDA expands mortality warnings on antipsychotic drugs. 2008. https://www.fdanews.com/articles/107752-fda-expands-mortality-warnings-on-antipsychotic-drugs. Accessed 20 Dec 2021.
- 40.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:1–16. doi: 10.1136/bmj.h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konovalov S, Muralee S, Tampi RR. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatrics. 2008;20:293–308. doi: 10.1017/S1041610207006540. [DOI] [PubMed] [Google Scholar]
- 42.International Psychogeriatric Association. IPA complete guides to behavioral and psychological symptoms of dementia (BPSD). 2012. https://www.ipa-online.org/publications/guides-to-bpsd. Accessed 20 Dec 2021.
- 43.Kales HC, Gitlin LN, Lyketsos CG. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a Multidisciplinary Expert Panel for the Detroit Expert Panel on the Assessment and Management of the Neuropsychiatric Symptoms of Dementia. J Am Geriatr Soc. 2014;62:762–769. doi: 10.1111/jgs.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tjia J, Hunnicutt JN, Herndon L, Blanks CR, Lapane KL, Wehry S. Association of a communication training program with use of antipsychotics in nursing homes. JAMA Intern Med. 2017;177:846–853. doi: 10.1001/jamainternmed.2017.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]