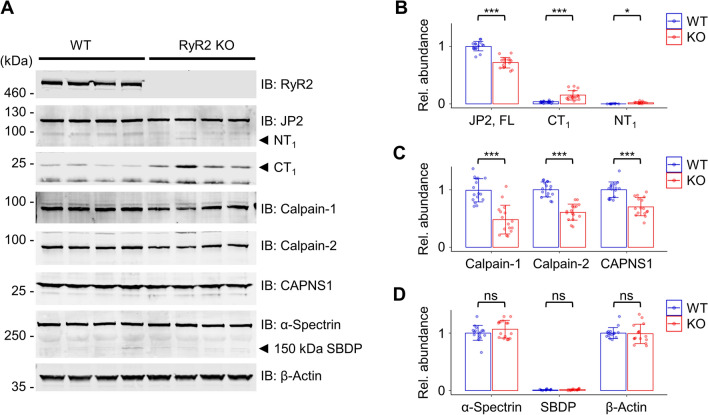
Figure 6.
RyR2 knockout reveals an increased Calpain-specific fragmentation of human JP2 in 2 months matured hiPSC-CMs. (A) Western blots of WT versus RyR2 knockout hiPSC-CM lysates detecting the proteins RyR2, JP2 versus its Calpain-specific cleavage fragments NT1 and CT1, Calpain-1, Calpain-2, CAPNS1, and the Calpain-specific substrate α-Spectrin versus its 150 kDa α-Spectrin cleavage product SBDP. The complete absence of RyR2-specific band signals confirmed its knockout in hiPSC-CMs. β-Actin was used as loading control. (B-D) Dot/bar plots summarizing the immunoblot data of WT versus RyR2 knockout hiPSC-CM lysates. (B) In contrast to WT hiPSC-CMs, in RyR2 knockout hiPSC-CMs the relative abundance of FL JP2 was significantly decreased, whereas the abundance of its NT1 and CT1 fragments was significantly increased. (C) Calpain-1, Calpain-2, and CAPNS1 showed significantly decreased levels in RyR2 knockout iPSC-CMs. (D) The abundance of the Calpain-specific substrate α-Spectrin and its breakdown product SBDP were not significantly changed, confirming human FL JP2 as the specific substrate of the increased Calpain activity in RyR2 KO hiPSC-CMs. Data were normalized to REVERT total protein stain (please refer to the methods section for additional details). Data are presented as mean ± SD (n = 4 biological replicates). Student’s t-test; *p < 0.05; ***p < 0.001.