Figure 3. The structure of the peptoid sidechain affects triple-helical propensity.
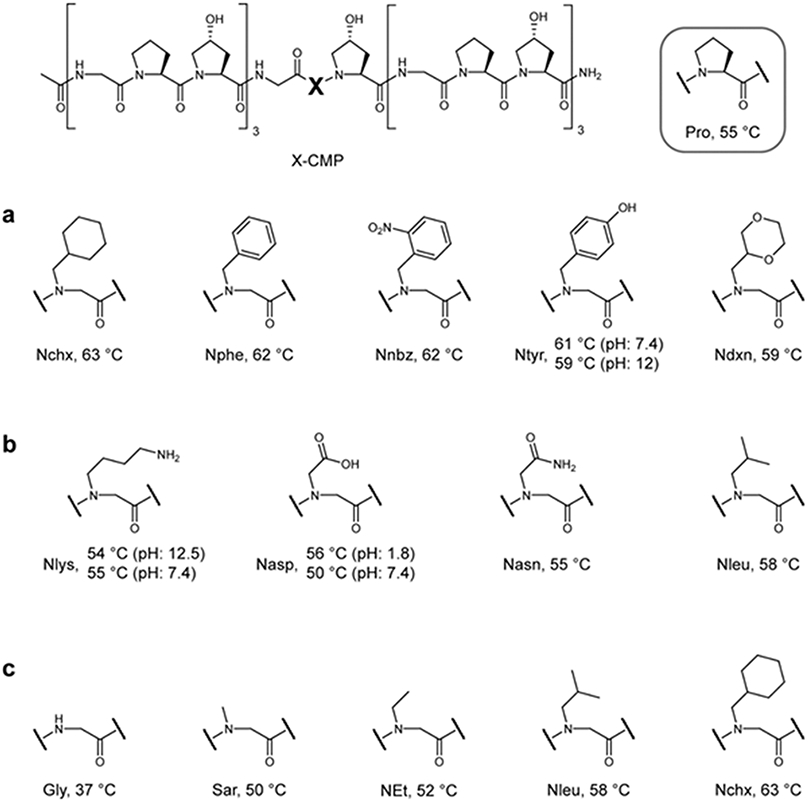
a, Tm values for a series of X-CMPs with N-Cα-substituted 6-membered rings. There is little difference in triple-helical stability among the aliphatic, aromatic, and hydrophilic rings, and every peptide had a Tm higher than the host: Pro-CMP. b, Although charged groups destabilized the triple-helix at physiological pH, when the sidechains were uncharged, both Nlys and Nasp, as well as the hydrophilic Nasn, were as stabilizing as Pro. c, A series of X-CMPs with aliphatic sidechains of increasing size showing concomitantly increasing Tm values, implying an robust steric effect. Although the hydrophobicity in this series increases as the aliphatic sidechains increase in size, according to series a and b, hydrophobicity has only a minor effect on stability. The results suggest that the tertiary amide structure N-gly (which hinders the α helix and β sheet conformations) and the size of the N-substituted sidechain play the most critical roles in stabilization of N-gly-CMP triple-helices.
