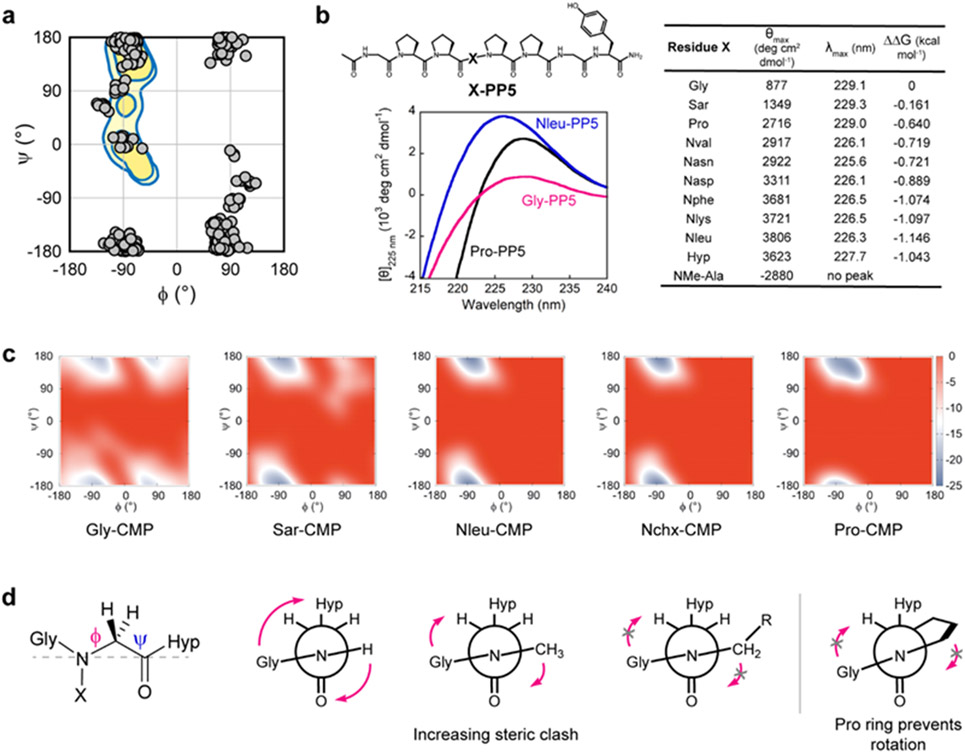
Figure 4. N-glys have a strong polyproline-II helix (PPII) propensity which stabilizes the X-CMP triple-helix.
a, A survey of dihedral angles from N-glys in small molecule crystal structures (from Cambridge Structure Database), showing their natural propensity for a left- or right-handed polyproline helix (watermark: regions populated by Pro). b, Structure of the PPII host-guest peptide X-PP5 and comparative CD spectra of Gly-PP5, Pro-PP5, and Nleu-PP5 showing PPII-characteristic signals near 228 nm. All N-glys, except Sar, had a θmax higher than Pro indicating peptoids’ strong PPII propensity. No peak was observed near 228 nm for NMe-Ala (Supporting Information Page S58). c, Metadynamics calculations of the free energy (kcal/mol) landscape of the guest residues in Gly-, Sar-, Nleu-, Nchx-, and Pro-CMPs. Each model yielded a native state consistent with the triple-helical and PPII conformations (−70°, 170°). However, peptides with small guest residues (Gly, Sar) showed a secondary non-PPII energy well. d, Newman-like projections of N-glys (grey dashed line: the line of sight for the projection). N-glys with larger sidechains may experience major steric clashes with their carbonyl group and have reduced conformational flexibility. This can help preorganize individual chains into a PPII conformation, thereby stabilizing the triple-helical assembly.