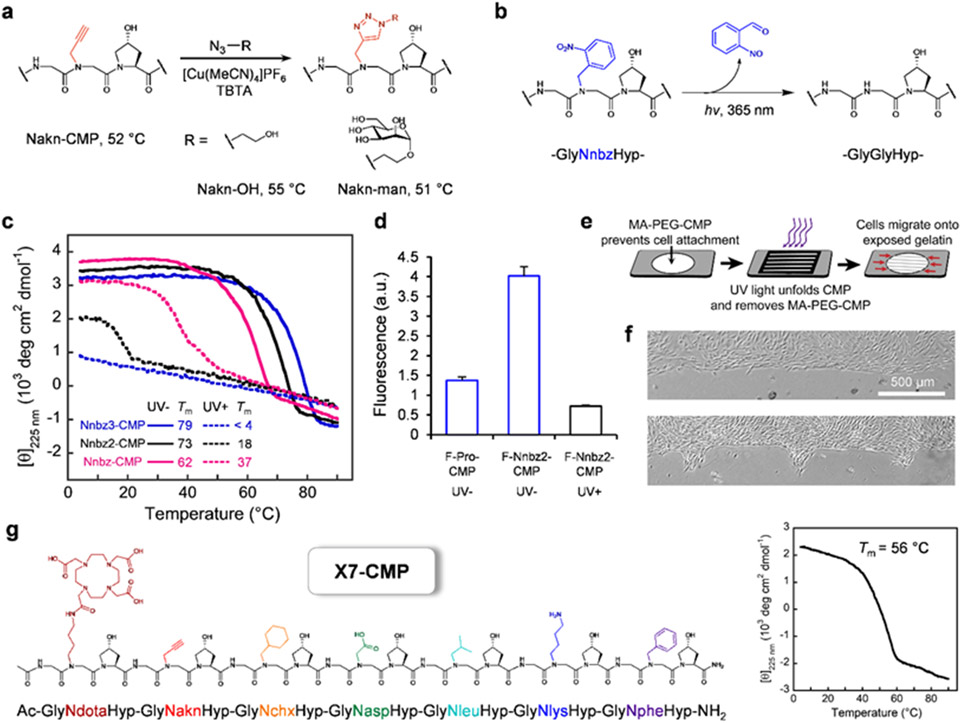
Figure 6. X-CMPs unlock unprecedented exotic functional designs.
a, Facile modification of the alkyne-sidechain through ‘click’ chemistry, forming spatially-demanding triazole units without destabilizing the triple-helix (Supplementary Discussion). b, Conversion of the triple-helix-stabilizing Nnbz to the destabilizing Gly via photo-cleavage of the N-o-nitrobenzyl group. c, CD melting curves showing widening of Tm difference before and after UV irradiation as more Nnbz residues replace Pro in the triple-helix (Table S7). d, Fluorescence of gelatin films treated with fluorescein-labeled X-CMPs, demonstrating F-Nnbz2-CMP’s higher affinity to gelatin, and its ability to unfold and unbind upon UV irradiation. e, Schematic of UV-patterning of a gelatin substrate enabled by Nnbz2-CMP conjugated to MA-PEG. f, Light micrographs of MDA-MB-231 cells grown on a gelatin film migrating from a defined boundary (top) into selected areas where the gelatin-bound MA-PEG-CMP had been removed by UV light through a photomask (1 d after UV, bottom). g, Pro-free X7-CMP hosting seven peptoid residues with diverse sidechain moieties showcasing a hyperstable triple-helical collagen peptide with the greatest sidechain diversity to date.