Table 2.
Discovery and evaluation of the Cu-catalyzed asymmetric cascade processa.
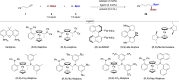 |
| Entry | Catalyst | Ligand | Solvent (mL) | Additive (equiv) | Yield (%)b | er c |
|---|---|---|---|---|---|---|
| 1 | Co(acac)2 | Xantphos | THF (0.2) | — | 76 | — |
| 2 | Co(acac)2 | (R,R)-Walphos | THF (0.2) | — | 33 | 39:61 |
| 3 | Co(acac)3 | (R,R)-Walphos | THF (0.2) | — | 28 | 35:65 |
| 4 | Cu(acac)2 | (R,R)-Walphos | THF (0.2) | — | 20 | 76:24 |
| 5 | Cu(acac)2 | (R,S)-Josiphos | THF (0.2) | — | 25 | 32:68 |
| 6 | Cu(acac)2 | (R)-tol-BINAP | THF (0.2) | — | 15 | 45:55 |
| 7 | Cu(acac)2 | (S,S)-Me-duphos | THF (0.2) | — | n.d. | — |
| 8 | Cu(acac)2 | (R,R)-Me-ferrocelane | THF (0.2) | — | n.d. | — |
| 9 | Cu(acac)2 | (R,R)-Walphos | Cyclohexane (1.0) | — | 33 | 88:12 |
| 10 | Cu(acac)2 | (R,R)-CH3-Walphos | Cyclohexane (1.0) | — | 16 | 72:28 |
| 11 | Cu(acac)2 | (R,R)-tBu-Walphos | Cyclohexane (1.0) | — | 17 | 62:38 |
| 12 | Cu(acac)2 | (R,R)-CF3-Walphos | Cyclohexane (1.0) | — | 18 | 86:14 |
| 13 | Cu(acac)2 | (R,R)-Nap-Walphos | THF (0.2) | — | 23 | 84:16 |
| 14d | Cu(acac)2 | (R,R)-Walphos | Cyclohexane (1.0) | PMHS (1.0) | 78 | 94:6 |
aAfter catalyst (4 mol%), ligand (4 mol%), HBdan (0.24 mmol) were mixed in 0.2 mL of THF at room temperature for 15 min, HBpin (0.3 mmol) and 1 (0.2 mmol) were added subsequently at room temperature, then the resulting mixture was stirred at ambient temperature for 24 h.
bIsolated yield.
cThe enantioselectivity was determined by Chiral HPLC.
dAfter catalyst (6 mol%), ligand (6 mol%), HBdan (0.24 mmol) and PMHS (1.0 equiv) were mixed in 1.0 mL of cyclohexane at room temperature for 10 min, HBpin (0.3 mmol) and 1 (0.2 mmol) were added subsequently at room temperature, then the resulting mixture was stirred at ambient temperature for 60 h.
