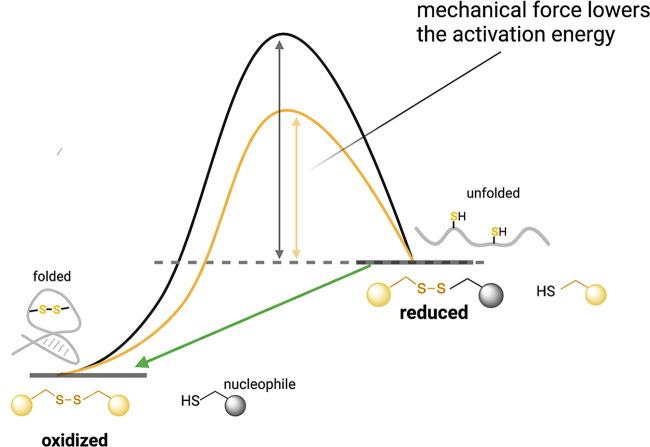
FIG 1.
Energy diagram showing the effect of mechanical force on the redox state of sulfhydryl groups and disulfide bonds and the consequences for protein unfolding/folding. Mechanical force lowers the activation energy (yellow curve) required to break protein disulfide bonds, a thermodynamically unfavorable reaction (ΔG > 0). Sufficient force puts the protein in a high-energy strained conformation, making it susceptible to nucleophilic attack. Due to the high-energy state of the products compared to the reactants, the reaction can be spontaneously reversible (green arrow), and the protein can revert to its original folded state (23). Note, however, that there are also cases where the application of force stabilizes a disulfide bond, effectively increasing the activation energy (32).