FIG 4.
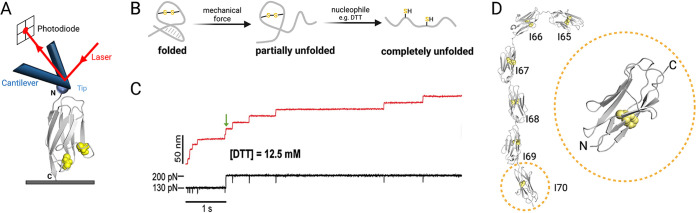
Mechanical force can induce protein unfolding shown in AFM studies with titin. (A) Schematic diagram of a force spectroscopy experiment where a functionalized AFM tip is used to probe the I27 domain of human cardiac muscle titin (PDB accession number 1TIT) (160). Cysteines in the protein are highlighted as yellow spheres, and the N and C termini are labeled. The protein is probed with a constant force or at a constant speed. When the tip encounters the protein, the tip starts to retract, pulling on the protein and exerting a force that causes the protein to unfold until it reaches a maximum (rupture) length. (B) Transition of a protein from a folded to a completely unfolded state. Mechanical force breaks hydrogen bonds and other intramolecular interactions within the protein, leaving the region enclosed by the disulfide bond still folded. In the presence of a reducing agent and force (or very high force alone), the protein completely unfolds, and the residues occluded by the original disulfide-bond-stabilized structure are fully exposed. (C) Double-pulse force clamp experiment for the I27 domain of titin with an engineered disulfide bond (22). An initial pulse of 130 pN causes an ~11-nm increase in the length of the protein, which corresponds to the partial unfolding of the protein. In the presence of dithiothreitol (DTT) (green arrow), and when the force is increased to 200 pN, steps of ~14 nm are observed, reflecting the unfolding of the region enclosed by the disulfide bond. (Republished from reference 22 with permission of the publisher.) (D) Crystal structure of the rabbit titin segment I65–I70 (PDB accession number 3B43) (161) marked by six tandem Ig domains. The protein contains 14 cysteines (yellow spheres) distributed among six Ig domains. The inset shows a magnified view of the I70 domain.