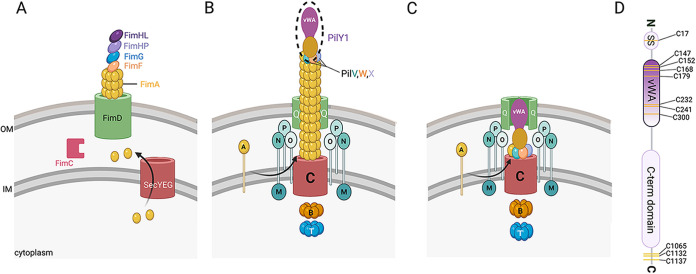
FIG 5.
The E. coli type I fimbriae and P. aeruginosa type IV pili (TFP). (A) FimA subunits are translocated from the cytoplasm into the periplasm through the SecYEG system. The FimC chaperone binds and stabilizes the major fimbria subunit FimA, facilitating the FimA protein attaining its native conformation, wherein FimA interacts with the outer membrane protein FimD. FimA is subsequently incorporated into the fimbriae. The adhesin FimH, together with the minor pilins FimF and FimG, is located at the distal tip of the pili. FimF and FimG are likely important for the integration of FimH into the pili (162, 163). FimH is an ~30-kDa protein consisting of two domains, an N-terminal mannoside-lectin binding domain (FimHL) and a C-terminal pilin domain (FimHP). Unlike the TFP, the type I fimbriae do not retract and are much shorter. (B) The TFP machinery: secretin (PilQ [green]), platform protein (PilC [red]), motors (PilB and PilT [orange and blue, respectively]), the alignment complex (PilMNOP [shades of aqua]), and pilus fiber (PilA monomers [yellow]). During extension, PilA monomers in the inner membrane (IM) are incorporated into the growing pilus, pushing the priming complex to the tip of the extended pilus, with PilY1 at the apex. (C) The TFP machinery with the pilus fully retracted. (D) Schematic showing the domain organization of PilY1. The signal sequence (SS), vWA domain, and C-terminal domain are shown. Yellow stripes represent the cysteines present in the protein. Seven of the 11 total cysteine residues are located in the vWA domain, with the signal sequence and the C-terminal region having 1 and 3 cysteine residues, respectively. PilY1 is ~120 kDa, and the vWA domain is ~26 kDa.