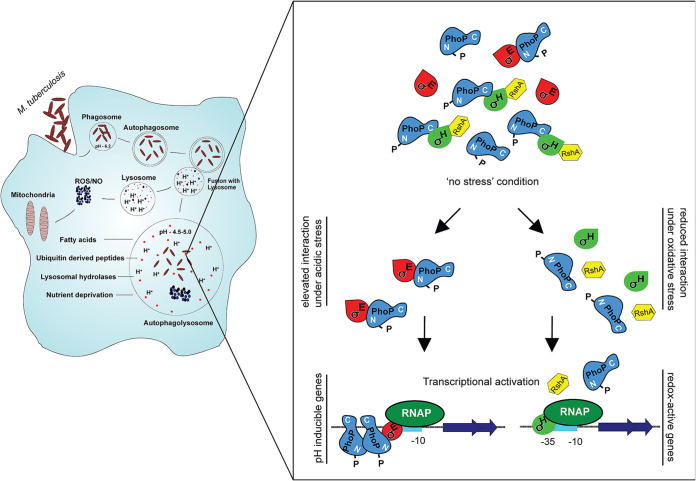
FIG 7.
Schematic model depicting low-pH- and redox-inducible mycobacterial gene expression. While PhoP restricts redox-inducible expression of mycobacterial thioredoxin genes under normal conditions, SigH is an activator of these genes under redox stress. Thus, under redox stress, the PhoP-SigH interaction is of no physiological consequence. Under normal conditions, thioredoxin genes are not influenced by either of the two regulators (PhoP or SigH). We propose that under normal conditions, perhaps there is interaction between PhoP, SigH, and RshA that effectively maintains the basal level of redox-active gene expression via blocking of SigH-dependent activation. However, under redox stress, dissociation of RhsA destabilizes the PhoP-SigH interaction, leading to release of SigH to activate redox-inducible gene expression. However, under acidic pH conditions, due to enhanced PhoP-SigE interaction, both PhoP and SigE are recruited within low-pH-inducible promoters of mycobacteria, and the transcription initiation promotes activation of acid-inducible genes. In summary, the PhoP-SigE interaction under acid stress contributes to pH homeostasis, whereas the reduced PhoP-SigH interaction under redox stress contributes to mycobacterial thiol redox homeostasis.