Fig. 3.
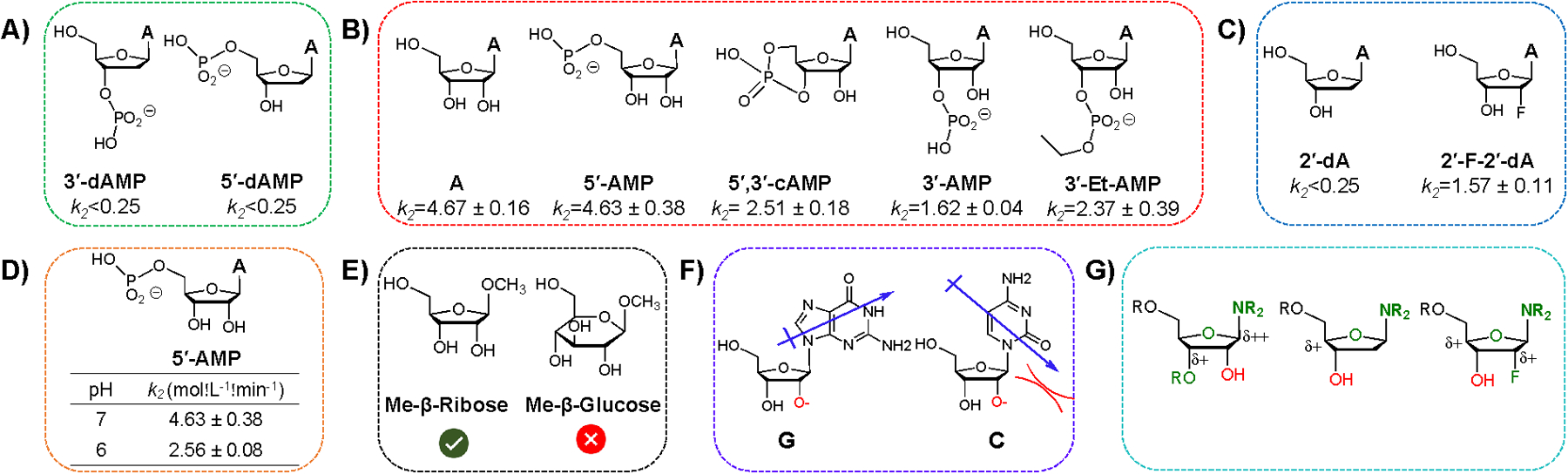
Additional compounds employed to study the mechanism of 2’-OH acylation with NAI. Second-order rate constants (k2) are shown for A-D.a For cases where k2<0.25, no acylation was observed, and this is an estimated maximum based on the smallest measurable peak area. A) Deoxynucleotide control compounds, which are unreactive; B) Nucleotides testing the role of neighbouring phosphate; C) Testing the role of inductive effects with a 2’-F group; D) Experiments at two pH values, showing rate enhancement at higher pH; E) Structures of sugar 1’-methylglycosides tested with NAI; F) Possible electronic effects on reactivity: hypothesised dipole effect on acidity of the 2’-OH. Base dipole in G electrostatically favors oxyanion, whereas the dipole in C disfavors it30; G) Schematic illustrating inductive effects of neighbouring O, N, F atoms (in green) on 2’/3’-OH (red) acidity. Conditions: 0.4 mM mononucleotide, 40 mM NAI in DMSO, containing 50 mM NaCl and 10 mM buffer (MOPS for pH 7, MES for pH 6), 23°C. DMSO content: 5% (v/v). Mean and standard deviation are shown from at least three independent experiments. For analytical data, see ESI Fig S11–24.
