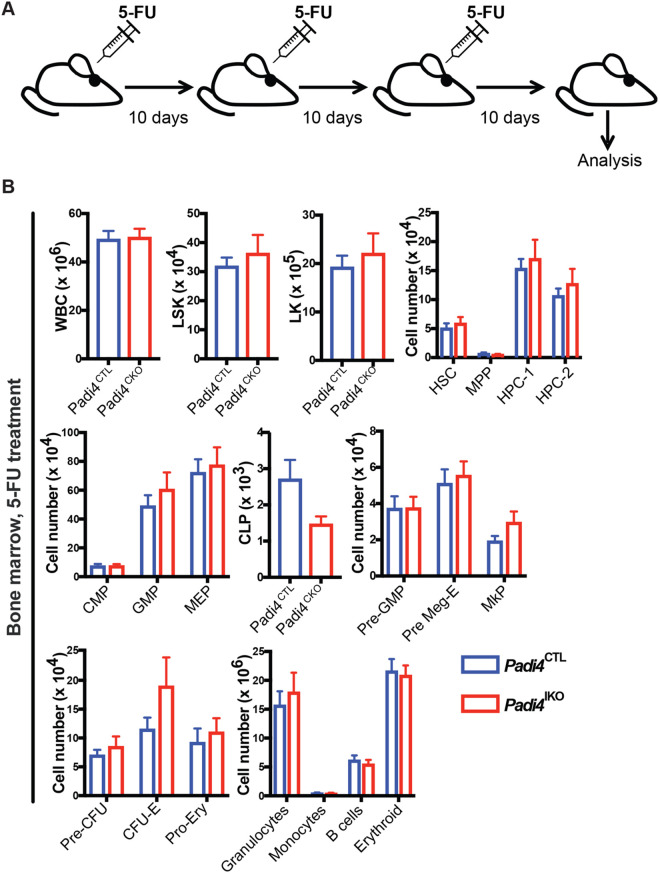
Fig. 4.
Assessment of Padi4 deletion the response to haematopoietic injury. (A) Experimental design of haematopoietic injury approach. Padi4CTL and Padi4CKO mice received a 3x 5-FU injections at 150 mg/kg 10 days apart and were analysed 10 days after the last administration. (B) Immunophenotypic analysis of Padi4CTL and Padi4CKO mice was performed 10 days after the final dose of 5-FU. Total number of cells in BM: WBC, LSK, and LK; HSC, MPP, HPC-1 and HPC-2 cells; myeloid, erythroid and lymphoid progenitor cells: CMP, GMP, MEP, CLP, Pre-GMP, Pre-MegE, MkP, Pre-CFU, CFU-E, Pro-Ery; differentiated granulocytes, monocytes, B cells, erythroid cells. Padi4CTL, n=21; Padi4CKO, n=18. All data are mean±s.e.m. *P<0.05; **P<0.01; ***P<0.001, ****P<0.0001 (Mann–Whitney U-test).